Melanoma is the deadliest type of skin cancer, due to its invasiveness and limited treatment efficacy. The main therapy for primary melanoma and solitary organ metastases is wide excision. Adjuvant therapy, such as chemotherapy and targeted therapies are mainly used for disseminated disease. Radiotherapy (RT) is a powerful treatment option used in more than 50% of cancer patients, however, conventional RT alone is unable to eradicate melanoma. Its general radioresistance is attributed to overexpression of repair genes in combination with cascades of biochemical repair mechanisms. A novel sophisticated technique based on synchrotron-generated, spatially fractionated RT, called Microbeam Radiation Therapy (MRT), has been shown to overcome these treatment limitations by allowing increased dose delivery. With MRT, a collimator subdivides the homogeneous radiation field into an array of co-planar, high-dose microbeams that are tens of micrometres wide and spaced a few hundred micrometres apart. Different preclinical models demonstrated that MRT has the potential to completely ablate tumours, or significantly improve tumour control while dramatically reducing normal tissue toxicity.
- radiotherapy
- melanoma
- immune response
- spatial fractionation
- microbeam radiotherapy
- synchrotron
- oncology
1. Introduction to Melanoma
2. Melanoma and Radiation Therapy
2.1. Radioresistance of Melanoma and Conventional RT as a Treatment Strategy
2.2. Spatially Fractionated RT Including Synchrotron-Generated MRT
3. MRT as a Novel Strategy for Treatment of Melanoma
A recent research interest of our group is exploring the efficacy of MRT for treating melanoma. An ad hoc preclinical B16F10 radioresistant melanoma model for testing a microbeam array was established in our laboratory [119][49]. In the very first study, it was clear that MRT elicits an extraordinary delay of tumour growth when compared to a BB irradiation or to un-irradiated controls [47]. Aside from the underlined mechanisms of vascular disruption and induction of cellular senescence, we demonstrated that MRT elicits superior tumour control due to the induction of a potent tumour immune response. Especially, MRT induces a significant increase of monocyte-attracting cytokines (MCP-1, MIP-1a, MIP-1b, RANTES), and IL-12p40 in the melanoma microenvironment [47]. MRT also promoted infiltration of macrophages, CD4+ and CD8+ T cells and NK cells, both in the periphery and within the irradiated tumour 5–12 days after treatment. This was observed in contrast to naïve or BB-irradiated tumours [47]. In another study, Fernandez-Palomo and colleagues compared the efficacy of delivering three fractions of 133 Gy MRT, administered in three consecutive days versus only one MRT fraction of 400 Gy in a single day [48]. Remarkably, after the temporally fractionated irradiation, 50% of melanomas underwent complete tumour remission. For an 18 months period, the mice showed no sign of tumour re-growth, and after they were sacrificed, immunohistochemical analysis of the tumoural vestige revealed a complete absence of melanoma cells and the presence of melanophages. Melanophages were described as very large melanin-laden cells, positive for macrophage markers [48]. The importance of this result is not only that radioresistant B16F10 melanoma does not lose sensitivity to the radiation treatment after several fractions, but also that there is no local tumour recurrence and metastasis for a long period of time. This could be explained by the presence of the anti-tumour abscopal effect specifically triggered by fractionated MRT. Therefore, this work offers a unique model to further study MRT-induced abscopal effects and is a starting point for optimizing a treatment protocol that could increase the rate of tumour remission above 50% in this preclinical model. In Table 1, we summarize findings in the field of MRT-induced tumour immune responses in melanoma and other cancers that were reported in this review. In Figure 2, we outline the advantages and disadvantages of conventional RT and MRT for the treatment of melanoma. In the case of MRT, the factors discussed above, such as the induction of the anti-tumour immune response, overcoming tumour radioresistance, and the ability to generate the anti-tumour abscopal effects, contribute to the treatment success in melanoma. We have yet to optimize the fractionation schedule on the basis of normal tissue tolerance and tumour response. In parallel, the success of MRT in melanoma treatment can be further boosted by exploiting the role of IR in the modulation of local and systemic immune processes. The treatment improvement will be based on enhancing the anti-tumour immune response in combination with MRT. This can be done directly by targeting immune checkpoints, or indirectly, by blocking the upstream of the innate (‘frontline’) immune response. The proof of concept of these treatment combinations will open a completely novel avenue for the treatment of radioresistant tumours beyond melanoma.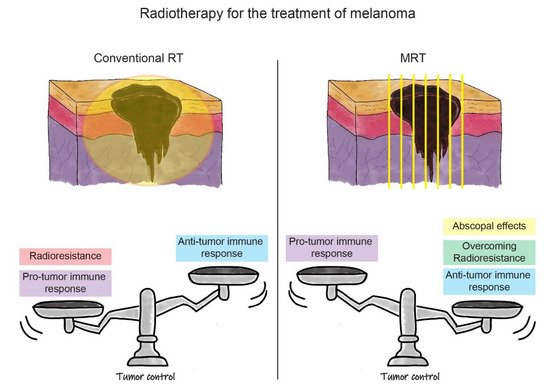
| Tumor Model | Assay Type | MRT Effects on Tumor Immune Response | Reference | |
| Glioblastoma in rat | Oligonucleotide microarray | Upregulation of genes associated with inflammation, NK or CD8+ T cells | Bouchet et al., 2013 [88] | Bouchet et al., 2013 [50] |
| Glioblastoma in rat | Oligonucleotide microarray | Upregulation of transcripts indicating the presence of DCs, monocytes, and macrophages | Bouchet et al., 2014 [89] | Bouchet et al., 2014 [51] |
| Glioblastoma in rat | IHC | Increase of infiltrated macrophages | Eling et al., 2021 [101] | Eling et al., 2021 [52] |
| Mammary EMT6.5 in mouse | Whole genome analysis | Upregulation of genes related to inflammation, IFN signalling, antigen presentation |
Sprung et al., 2012 [87] | Sprung et al., 2012 [53] |
| Mammary EMT6.5 cell line | Whole genome analysis | Upregulation of pathways involved in inflammation and lymphocyte activation | Yang et al., 2014 [90] | Yang et al., 2014 [54] |
| Mammary EMT6.5 in mouse | Flow cytometry, IHC | Decrease in tumour-associated macrophages and neutrophils; increase of infiltrated T cells |
Yang et al., 2019 [96] | Yang et al., 2019 [55] |
| Melanoma B16F10 in mouse | Cytokine BioPlex analysis, IHC | Increase of monocyte-attracting cytokines; Increase of infiltrated macrophages, CD4+ and CD8+ T cells, NK cells | Potez et al., 2019 [47] | |
| Melanoma B16F10 in mouse | IHC | Presence of melanophages at the place of tumor cells and absence of metastasis up to 18 months post-treatment | Fernandez-Palomo et al., 2020 [48] |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30.
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 2019, 144, 1941–1953.
- Usher-Smith, J.A.; Emery, J.; Kassianos, A.P.; Walter, F.M. Risk Prediction Models for Melanoma: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1450–1463.
- Thomas, N.E.; Kricker, A.; Waxweiler, W.T.; Dillon, P.M.; Busman, K.J.; From, L.; Groben, P.A.; Armstrong, B.K.; Anton-Culver, H.; Gruber, S.B.; et al. Comparison of Clinicopathologic Features and Survival of Histopathologically Amelanotic and Pigmented Melanomas: A Population-Based Study. JAMA Dermatol. 2014, 150, 1306–1314.
- Kuk, D.; Shoushtari, A.N.; Barker, C.A.; Panageas, K.S.; Munhoz, R.R.; Momtaz, P.; Ariyan, C.E.; Brady, M.S.; Coit, D.G.; Bogatch, K.; et al. Prognosis of Mucosal, Uveal, Acral, Nonacral Cutaneous, and Unknown Primary Melanoma From the Time of First Metastasis. Oncologist 2016, 21, 848–854.
- Kunte, C.; Geimer, T.; Baumert, J.; Konz, B.; Volkenandt, M.; Flaig, M.; Ruzicka, T.; Berking, C.; Schmid-Wendtner, M.-H. Prognostic Factors Associated with Sentinel Lymph Node Positivity and Effect of Sentinel Status on Survival: An Analysis of 1049 Patients with Cutaneous Melanoma. Melanoma Res. 2010, 20, 330–337.
- Shain, A.H.; Bastian, B.C. From Melanocytes to Melanomas. Nat. Rev. Cancer 2016, 16, 345–358.
- Vyas, R.; Selph, J.; Gerstenblith, M.R. Cutaneous Manifestations Associated with Melanoma. Semin. Oncol. 2016, 43, 384–389.
- Keung, E.Z.; Gershenwald, J.E. The Eighth Edition American Joint Committee on Cancer (AJCC) Melanoma Staging System: Implications for Melanoma Treatment and Care. Expert Rev. Anticancer Ther. 2018, 18, 775–784.
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma Staging: Evidence-Based Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J. Clin. 2017, 67, 472–492.
- Hayes, A.J.; Maynard, L.; Coombes, G.; Newton-Bishop, J.; Timmons, M.; Cook, M.; Theaker, J.; Bliss, J.M.; Thomas, J.M.; UK Melanoma Study Group; et al. Wide versus Narrow Excision Margins for High-Risk, Primary Cutaneous Melanomas: Long-Term Follow-up of Survival in a Randomised Trial. Lancet Oncol. 2016, 17, 184–192.
- Young, S.E.; Martinez, S.R.; Faries, M.B.; Essner, R.; Wanek, L.A.; Morton, D.L. Can Surgical Therapy Alone Achieve Long-Term Cure of Melanoma Metastatic to Regional Nodes? Cancer J. 2006, 12, 207–211.
- Leiter, U.; Stadler, R.; Mauch, C.; Hohenberger, W.; Brockmeyer, N.; Berking, C.; Sunderkötter, C.; Kaatz, M.; Schulte, K.-W.; Lehmann, P.; et al. Complete Lymph Node Dissection versus No Dissection in Patients with Sentinel Lymph Node Biopsy Positive Melanoma (DeCOG-SLT): A Multicentre, Randomised, Phase 3 Trial. Lancet Oncol. 2016, 17, 757–767.
- Takahashi, J.; Nagasawa, S. Immunostimulatory Effects of Radiotherapy for Local and Systemic Control of Melanoma: A Review. Int. J. Mol. Sci. 2020, 21, 9324.
- Dewey, D.L. The Radiosensitivity of Melanoma Cells in Culture. BJR 1971, 44, 816–817.
- Barranco, S.C.; Romsdahl, M.M.; Humphrey, R.M. The Radiation Response of Human Malignant Melanoma Cells Grown in Vitro. Cancer Res. 1971, 31, 830–833.
- Kauffmann, A.; Rosselli, F.; Lazar, V.; Winnepenninckx, V.; Mansuet-Lupo, A.; Dessen, P.; van den Oord, J.J.; Spatz, A.; Sarasin, A. High Expression of DNA Repair Pathways Is Associated with Metastasis in Melanoma Patients. Oncogene 2008, 27, 565–573.
- Wu, L.; Hu, Z.; Huang, Y.; Yu, Y.; Liang, W.; Zheng, Q.; Huang, X.; Huang, Y.; Lu, X.; Zhao, Y. Radiation Changes the Metabolic Profiling of Melanoma Cell Line B16. PLoS ONE 2016, 11, e0162917.
- Rofstad, E.K. Radiation Biology of Malignant Melanoma. Acta Radiol. Oncol. 1986, 25, 1–10.
- Stevens, G.; McKay, M.J. Dispelling the Myths Surrounding Radiotherapy for Treatment of Cutaneous Melanoma. Lancet Oncol. 2006, 7, 575–583.
- Chavaudra, N.; Guichard, M.; Malaise, E.P. Hypoxic Fraction and Repair of Potentially Lethal Radiation Damage in Two Human Melanomas Transplanted into Nude Mice. Radiat. Res. 1981, 88, 56–68.
- Rofstad, E.K.; Brustad, T. Tumour Growth Delay Following Single Dose Irradiation of Human Melanoma Xenografts. Correlations with Tumour Growth Parameters, Vascular Structure and Cellular Radiosensitivity. Br. J. Cancer 1985, 51, 201–210.
- Matchuk, O.N.; Zamulaeva, I.A.; Kovalev, O.A.; Saenko, A.S. Radioresistance mechanisms of side population cells in mouse melanoma cell line B16. Tsitologiia 2013, 55, 553–559.
- Bastiaannet, E.; Beukema, J.C.; Hoekstra, H.J. Radiation Therapy Following Lymph Node Dissection in Melanoma Patients: Treatment, Outcome and Complications. Cancer Treat. Rev. 2005, 31, 18–26.
- Keenan, L.G.; O’Sullivan, S.; Glynn, A.; Higgins, M.; Flavin, A.; Brennan, S. Clinical Review of Treatment Outcomes and Patterns of Failure with Adjuvant Radiotherapy in Node-Positive Malignant Melanoma. J. Med. Imaging Radiat. Oncol. 2017, 61, 258–262.
- Eddy, K.; Chen, S. Overcoming Immune Evasion in Melanoma. Int. J. Mol. Sci. 2020, 21, 8984.
- Habermalz, H.J.; Fischer, J.J. Radiation Therapy of Malignant Melanoma: Experience with High Individual Treatment Doses. Cancer 1976, 38, 2258–2262.
- Lugade, A.A.; Moran, J.P.; Gerber, S.A.; Rose, R.C.; Frelinger, J.G.; Lord, E.M. Local Radiation Therapy of B16 Melanoma Tumors Increases the Generation of Tumor Antigen-Specific Effector Cells That Traffic to the Tumor. J. Immunol. 2005, 174, 7516–7523.
- Shi, W. Role for Radiation Therapy in Melanoma. Surg. Oncol. Clin. N. Am. 2015, 24, 323–335.
- Mohiuddin, M.; Stevens, J.H.; Reiff, J.E.; Huq, M.S.; Suntharalingam, N. Spatially Fractionated (GRID) Radiation for Palliative Treatment of Advanced Cancer. Radiat. Oncol. Investig. 1996, 4, 41–47.
- Mohiuddin, M.; Fujita, M.; Regine, W.F.; Megooni, A.S.; Ibbott, G.S.; Ahmed, M.M. High-Dose Spatially-Fractionated Radiation (GRID): A New Paradigm in the Management of Advanced Cancers. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 721–727.
- Yan, W.; Khan, M.K.; Wu, X.; Simone, C.B.; Fan, J.; Gressen, E.; Zhang, X.; Limoli, C.L.; Bahig, H.; Tubin, S.; et al. Spatially Fractionated Radiation Therapy: History, Present and the Future. Clin. Transl. Radiat. Oncol. 2019, 20, 30–38.
- Shirato, H.; Gupta, N.K.; Jordan, T.J.; Hendry, J.H. Lack of Late Skin Necrosis in Man after High-Dose Irradiation Using Small Field Sizes: Experiences of Grid Therapy. Br. J. Radiol. 1990, 63, 871–874.
- Wu, X.; Ahmed, M.M.; Wright, J.; Gupta, S.; Pollack, A. On Modern Technical Approaches of Three-Dimensional High-Dose Lattice Radiotherapy (LRT). Cureus 2010, 2.
- Wu, X.; Perez, N.C.; Zheng, Y.; Li, X.; Jiang, L.; Amendola, B.E.; Xu, B.; Mayr, N.A.; Lu, J.J.; Hatoum, G.F.; et al. The Technical and Clinical Implementation of LATTICE Radiation Therapy (LRT). Radiat. Res. 2020, 194, 737–746.
- Prezado, Y.; Fois, G.R. Proton-Minibeam Radiation Therapy: A Proof of Concept. Med. Phys. 2013, 40, 031712.
- Prezado, Y.; Jouvion, G.; Guardiola, C.; Gonzalez, W.; Juchaux, M.; Bergs, J.; Nauraye, C.; Labiod, D.; De Marzi, L.; Pouzoulet, F.; et al. Tumor Control in RG2 Glioma-Bearing Rats: A Comparison Between Proton Minibeam Therapy and Standard Proton Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 266–271.
- Dilmanian, F.A.; Zhong, Z.; Bacarian, T.; Benveniste, H.; Romanelli, P.; Wang, R.; Welwart, J.; Yuasa, T.; Rosen, E.M.; Anschel, D.J. Interlaced X-Ray Microplanar Beams: A Radiosurgery Approach with Clinical Potential. Proc. Natl. Acad. Sci. USA 2006, 103, 9709–9714.
- Prezado, Y.; Sarun, S.; Gil, S.; Deman, P.; Bouchet, A.; Le Duc, G. Increase of Lifespan for Glioma-Bearing Rats by Using Minibeam Radiation Therapy. J. Synchrotron Radiat. 2012, 19, 60–65.
- Prezado, Y.; Dos Santos, M.; Gonzalez, W.; Jouvion, G.; Guardiola, C.; Heinrich, S.; Labiod, D.; Juchaux, M.; Jourdain, L.; Sebrie, C.; et al. Transfer of Minibeam Radiation Therapy into a Cost-Effective Equipment for Radiobiological Studies: A Proof of Concept. Sci. Rep. 2017, 7, 17295.
- Hadsell, M.; Zhang, J.; Laganis, P.; Sprenger, F.; Shan, J.; Zhang, L.; Burk, L.; Yuan, H.; Chang, S.; Lu, J.; et al. A First Generation Compact Microbeam Radiation Therapy System Based on Carbon Nanotube X-Ray Technology. Appl. Phys. Lett. 2013, 103, 183505.
- Fernandez-Palomo, C.; Fazzari, J.; Trappetti, V.; Smyth, L.; Janka, H.; Laissue, J.; Djonov, V. Animal Models in Microbeam Radiation Therapy: A Scoping Review. Cancers 2020, 12, 527.
- Eling, L.; Bouchet, A.; Nemoz, C.; Djonov, V.; Balosso, J.; Laissue, J.; Bräuer-Krisch, E.; Adam, J.F.; Serduc, R. Ultra High Dose Rate Synchrotron Microbeam Radiation Therapy. Preclinical Evidence in View of a Clinical Transfer. Radiother. Oncol. 2019, 139, 56–61.
- Bräuer-Krisch, E.; Adam, J.-F.; Alagoz, E.; Bartzsch, S.; Crosbie, J.; DeWagter, C.; Dipuglia, A.; Donzelli, M.; Doran, S.; Fournier, P.; et al. Medical Physics Aspects of the Synchrotron Radiation Therapies: Microbeam Radiation Therapy (MRT) and Synchrotron Stereotactic Radiotherapy (SSRT). Phys. Med. 2015, 31, 568–583.
- Marcu, L.G.; Bezak, E.; Peukert, D.D.; Wilson, P. Translational Research in FLASH Radiotherapy-From Radiobiological Mechanisms to In Vivo Results. Biomedicines 2021, 9, 181.
- Montay-Gruel, P.; Acharya, M.M.; Petersson, K.; Alikhani, L.; Yakkala, C.; Allen, B.D.; Ollivier, J.; Petit, B.; Jorge, P.G.; Syage, A.R.; et al. Long-Term Neurocognitive Benefits of FLASH Radiotherapy Driven by Reduced Reactive Oxygen Species. Proc. Natl. Acad. Sci. USA 2019, 116, 10943–10951.
- Potez, M.; Fernandez-Palomo, C.; Bouchet, A.; Trappetti, V.; Donzelli, M.; Krisch, M.; Laissue, J.; Volarevic, V.; Djonov, V. Synchrotron Microbeam Radiation Therapy as a New Approach for the Treatment of Radioresistant Melanoma: Potential Underlying Mechanisms. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1126–1136.
- Fernandez-Palomo, C.; Trappetti, V.; Potez, M.; Pellicioli, P.; Krisch, M.; Laissue, J.; Djonov, V. Complete Remission of Mouse Melanoma after Temporally Fractionated Microbeam Radiotherapy. Cancers 2020, 12, 2656.
- Potez, M.; Trappetti, V.; Bouchet, A.; Fernandez-Palomo, C.; Güç, E.; Kilarski, W.W.; Hlushchuk, R.; Laissue, J.; Djonov, V. Characterization of a B16-F10 Melanoma Model Locally Implanted into the Ear Pinnae of C57BL/6 Mice. PLoS ONE 2018, 13, e0206693.
- Bouchet, A.; Sakakini, N.; El Atifi, M.; Le Clec’h, C.; Brauer, E.; Moisan, A.; Deman, P.; Rihet, P.; Le Duc, G.; Pelletier, L. Early Gene Expression Analysis in 9L Orthotopic Tumor-Bearing Rats Identifies Immune Modulation in Molecular Response to Synchrotron Microbeam Radiation Therapy. PLoS ONE 2013, 8, e81874.
- Bouchet, A.; Sakakini, N.; El Atifi, M.; Le Clec’h, C.; Bräuer-Krisch, E.; Rogalev, L.; Laissue, J.; Rihet, P.; Le Duc, G.; Pelletier, L. Identification of AREG and PLK1 Pathway Modulation as a Potential Key of the Response of Intracranial 9L Tumor to Microbeam Radiation Therapy. Int. J. Cancer. J. Int. Cancer 2014. in print.
- Eling, L.; Bouchet, A.; Ocadiz, A.; Adam, J.-F.; Kershmiri, S.; Elleaume, H.; Krisch, M.; Verry, C.; Laissue, J.A.; Balosso, J.; et al. Unexpected Benefits of Multiport Synchrotron Microbeam Radiation Therapy for Brain Tumors. Cancers 2021, 13, 936.
- Sprung, C.N.; Yang, Y.; Forrester, H.B.; Li, J.; Zaitseva, M.; Cann, L.; Restall, T.; Anderson, R.L.; Crosbie, J.C.; Rogers, P.A.W. Genome-Wide Transcription Responses to Synchrotron Microbeam Radiotherapy. Radiat. Res. 2012, 178, 249.
- Yang, Y.; Crosbie, J.C.; Paiva, P.; Ibahim, M.; Stevenson, A.; Rogers, P.A.W. In Vitro Study of Genes and Molecular Pathways Differentially Regulated by Synchrotron Microbeam Radiotherapy. Rare 2014, 182, 626–639.
- Yang, Y.; Swierczak, A.; Ibahim, M.; Paiva, P.; Cann, L.; Stevenson, A.W.; Crosbie, J.C.; Anderson, R.L.; Rogers, P.A.W. Synchrotron Microbeam Radiotherapy Evokes a Different Early Tumor Immunomodulatory Response to Conventional Radiotherapy in EMT6.5 Mammary Tumors. Radiother. Oncol. 2019, 133, 93–99.
