Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Joseph Biggs.
In 2016, a Zika virus outbreak in Brazil gained global prominence due to it coinciding with a sudden spike in birth abnormalities that was later attributed to pregnant women being infected with the virus. Today zika transmission patterns, particularly at sub-national levels, remain poorly described. Here we described evidence of widespread Zika transmission across the Philippines.
- Zika
- dengue
- serology
- diagnostics
1. Introduction
Zika is a flavivirus predominantly transmitted by Aedes mosquitoes which typically causes asymptomatic, or occasionally mild self-limited symptomatic, infections in humans. Consequently, previous global Zika outbreaks during the 20th century were underreported, and the disease was of limited public health concern [1]. In 2016, Zika gained global prominence due to an outbreak in Brazil coinciding with an unprecedented rise in severe birth abnormalities [2]. Subsequent studies linked Zika virus infections with Guillain-Barré syndrome [3] and microcephaly in infants [4]. Today, heightened surveillance operations report evidence of autochthonous Zika transmission in approximately 87 countries [1]. However, population exposure rates and transmission patterns at subnational levels remain poorly characterized, at least partially because of the difficulties in distinguishing Zika from other flavivirus infections [5,6,7][5][6][7].
Similar to other flaviviruses, including dengue, Zika virus (ZIKV) infection in humans is characterized by an initial viremic, followed by an immunogenic phase. A few days post-infection, viremia increases rapidly in hosts, during which time viral RNA is detectable in the blood for a few days [8,9][8][9]. Shortly after this peak in viremia, hosts elicit IgM antibodies that likely persist for months post-infection [10]. Approximately a week after the peak in viremia, hosts mount a long-term IgG antibody response that offers protection from successive Zika infections and is thought to be detectable for decades [11]. In contrast, for flaviviral infections caused by dengue virus (DENV), the existence of four serologically distinct serotypes (DENV1-4) means that immunity only offers protection from subsequent homologous, not heterologous, serotypes enabling post-primary (secondary, tertiary, or quaternary) dengue infections [12]. During a secondary infection, previously elicited IgG no longer neutralizes, but instead cross-reacts and surges with the new serotype to trigger the antibody-dependent enhancement (ADE) of viral replication. Increased virus replication during a secondary infection is thought to result in more severe disease because host-elicited cytokine storms can trigger vascular leakage [12,13,14][12][13][14]. Interestingly, the extensive structural and antigenic homology between ZIKV and DENV has generated speculation as to whether cross-reactive IgG responses from a Zika infection can result in the enhancement of dengue [15]. Indeed, a recent cohort study conducted in Nicaragua revealed that infection by Zika enhances the future risk of severe disease in subsequent DENV-2 infections, comparable to a previous heterologous dengue serotype, suggesting possible ADE mechanisms [16]. A pattern also found in vivo when rhesus macaques, previously infected with ZIKV, experienced higher viremia and proinflammatory cytokines during a subsequent DENV-2 infection compared to those previously uninfected with ZIKV [17]. In contrast, a Brazilian study reported a decline in dengue infections following a Zika outbreak, eluding to cross-protection, not enhancement [18]. However, given that cross-protective ZIKV IgG antibodies wane over time [19], the remaining cross-reactive antibodies may facilitate adverse ADE mechanisms later in life.
With disease presentation largely asymptomatic and with a short window of viral detection, serological diagnosis is crucial for capturing Zika cases. However, cross-reactive antibody responses between ZIKV and DENV present a challenge for differential diagnosis. Numerous commercial serological diagnostic tests have been developed recently, including the Euroimmun (Lübeck, Germany) indirect IgM and IgG ELISAs (enzyme-linked immunosorbent assays), which state that the kits are highly specific for Zika [20]. Recent studies have utilized Euroimmun and have reported Zika specificity >90% [21,22][21][22], although the test subjects often included small groups of infected travelers who resided outside flavivirus-endemic countries. More recently, however, the accuracy of these commercial tests has been brought into question by studies in flavivirus-endemic regions, including Salvador (Brazil) [23], Rio de Janerio (Brazil) [24], and Carabobo (Venezuela) [25]. Studies have revealed that IgM ELISAs have adequate specificity, yet poor sensitivity for capturing active ZIKV infections. Conversely, studies have shown that ZIKV IgG ELISAs have favorable sensitivities, yet variable specificities in distinguishing Zika from dengue infections. Moreover, one group demonstrated that ZIKV IgG kits reasonably differentiated Zika infections from primary dengue infections, yet not secondary dengue infections [24], which may be a potential consequence of ZIKV IgG simply cross-reacting with pre-circulating IgG during a secondary infection elicited from either a prior dengue or Zika infection. This corresponds to findings described by the authors of [26], who evaluated a novel immunomagnetic assay for ZIKV and found that ZIKV IgG was elevated among secondary DENV infections, but not primary DENV infections. Further understanding into how ZIKV IgG responses change during the acute stage of a dengue infection, and of those reporting without active dengue infections, may help distinguish whether patients have experienced prior dengue or Zika infections.
In the Philippines, Zika transmission remains poorly understood. Prior to 2016, isolated reports of confirmed Zika infections among non-travelling individuals in two cities—Quezon City in 2010 [27] and Cebu City in 2012 [28]—eluded to autochthonous transmission rather than imported Zika. Then, in 2016, a total of 47 non-travelling, PCR-confirmed Zika cases were detected after enhanced surveillance operations incorporated fever, rash, arthralgia and conjunctivitis into their case definition [29]. Considering that Zika cases are often asymptomatic and symptomatic infections resemble other co-endemic febrile illnesses, relying on passive case reports likely underestimates the true burden of disease. In Thailand, a recent study revealed evidence of persistent Zika transmission throughout the whole country [6].
2. Data Description
We successfully assayed 997/1000 suspected dengue case reports who visited 102 DRUs situated in all 17 regions of the Philippines during 2016 (Figure 1) (Supplementary Materials Table S1). The demographic characteristics of the sampled population are shown in Supplementary Materials Table S2. Overall, most cases were aged between 6 and 15 years (43.3%, 432/997), reported between disease day four and five (51.4%, 512/997) and presented with warning signs of dengue (54.4% 542/997). Of those who reported with suspected dengue, we estimated that 80.1% (794/991) presented with an active DENV infection (PCR+ or IgM+). Among active dengue infections, we classified 23.8% (189/794) as primary and 76.2% (605/794) as post-primary infections.
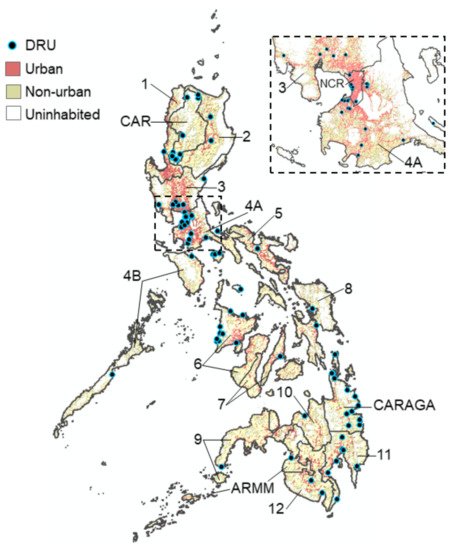

Figure 1. Map of the Philippines showing the location of the 102 DRUs where dengue patients were sampled from across all 17 regions during 2016. Urban zones: >1500 persons per km2. Non-urban zones: <1500 persons per km2.
3. ZIKV and DENV Cross-Reactive Antibody Responses
The majority of the sampled population reported with elevated dengue antibody responses, with 70.2% (696/991) DENV IgM+ and 80.7% (800/991) DENV IgG+ (Figure 2A). This infers a high degree of short- and long-term exposure to dengue among the sampled population.
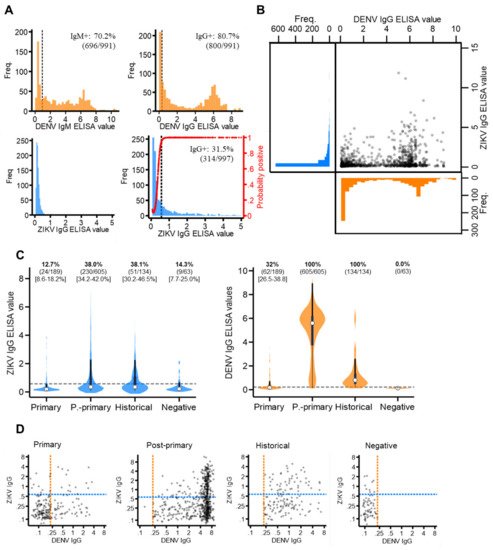

Figure 2. ZIKV and DENV antibody responses among the study population. (A) Distribution of ZIKV and DENV IgM and IgG antibody responses (ELISA values) among the study population. Red line: probability of being ZIKV IgG seropositive according to the mixture model. (B) Scatterplot of ZIKV versus DENV IgG ELISA values among the study population. (C) Violin plots of ZIKV and DENV IgG ELISA values among those classified as DENV primary, post-primary, historical and negative. White circles: median, thick black bar: IQR. Grey dash: IgG seroprevalence thresholds (ZIKV IgG: 0.57 ELISA values, DENV IgG: 0.22 ELISA values). (D) Scatterplots of ZIKV versus DENV IgG ELISA values among DENV primary, post-primary, historical and negative cases plotted on a log scale. Orange dash: DENV IgG seroprevalence threshold (0.22 ELISA values). Blue dash: ZIKV IgG seroprevalence threshold (0.57 ELISA values).
For ZIKV IgM and IgG, seroprevalence thresholds were determined using mixture models. For ZIKV IgG, a two-component, as opposed to a one-component model, best fit the data (AIC difference: −284.2) (Supplementary Materials Figure S1). This generated a new ZIKV IgG seroprevalence threshold of 0.57 ELISA values, which resulted in 31.5% (314/997) of the study population having ZIKV IgG exposure (Figure 2A). For ZIKV IgM, a mixture model fitted with just one, instead of two, components best fit the data (AIC difference: +159.9), as most of the study population reported with very low ZIKV IgM levels. We were therefore unable to determine IgM seroprevalence. Thus, we concluded that, despite a proportion of the study reporting with long-term exposure, no evidence of recent Zika exposure was found in the study population.
Cross-reacting IgG responses between Zika and dengue are shown in Figure 2B. Among those with elevated (seropositive) DENV IgG, most reported with low levels of ZIKV IgG (63.1% (505/800) ZIKV IgG-). Contrastingly, among those with elevated (seropositive) ZIKV IgG, nearly all reported with elevated DENV IgG responses (93.6% (295/314) DENV IgG positive). This suggests the Panbio IgG ELISA kits detected IgG from either ZIKV or DENV, while Euroimmun IgG ELISA kits were more specific to ZIKV antibodies. Notably, after stratifying by DENV immune status, we found a significantly higher proportion of post-primary (38.0% (95%CI: 4.2–42.0%)) and historical (38.1% (95%CI: 30.2–46.5)) cases were classified as ZIKV IgG-positive compared to primary (12.7% (95%CI: 8.6–18.2%)) and negative (14.3% (95%CI: 7.7–25.0%)) dengue cases. Moreover, despite post-primary cases experiencing higher DENV IgG responses (median: 5.6 (IQR: 3.7–6.2)) than historical cases (median: 0.8 (IQR: 0.5–1.4)), the same proportion of these cases reported ZIKV IgG-seropositive (38%) (Figure 2C). Therefore, if ZIKV IgG was elevated solely due to elevated DENV IgG, then a higher proportion of post-primary than historical cases would be ZIKV IgG positive. However, this was not observed. Last, despite negative cases (clinically misdiagnosed dengue cases) being DENV IgG-negative, 14.3% (9/63) reported with distinctly elevated ZIKV IgG levels (ZIKV IgG-seropositive), which, notably, cannot be attributed to ZIKV/DENV cross-reactivity (Figure 2D).
4. ZIKV Immunoepidemiology in the Philippines
We next investigated how the progression of an active DENV infection influenced ZIKV IgG responses in the study population. Among those reporting with primary, historical or negative DENV infections, we observed no difference in the proportion ZIKV IgG positive by day of disease (Supplementary Materials Figure S2). This suggests that ZIKV IgG levels remained stable during the acute stage of primary DENV infections and in those reporting with historical or negative DENV infections. In contrast, ZIKV IgG positivity significantly increased by disease day among those reporting with post-primary DENV infections, particularly those under 10 years of age (Figure 3A). In total, 14.3% (95%CI: 0.00–35.3%) of post-primary infections under 10 years of age were ZIKV IgG-seropositive between disease days 0–1, which increased to 53.8% (95%CI: 44.7–62.9%) between disease days 4–5. Interestingly, this increasing trend was not observed among older post-primary infections (Figure 3A), but was observed among post-primary infections stratified by serotype, although these differences did not reach statistical significance (Supplementary Materials Figure S1). Together, this infers that ZIKV IgG surges during the acute phase of a post-primary DENV infection, particularly among those who are younger. We then explored how age impacted ZIKV IgG seroprevalence among the study population and found contrasting age-ZIKV IgG seropositivity trends between post-primary and historical DENV cases (Figure 3B). ZIKV IgG seroprevalence decreased with increasing age among post-primary DENV infections, whereby 54.8% (95%CI: 43.1–66.5%) of those under 5 years of age were ZIKV IgG-seropositive, which gradually decreased to 26.8% (95%CI 14.8–38.8%) among those aged between 21–25 years. ZIKV IgG seroprevalence appeared to increase with age among historical cases, although this was not statistically significant (Figure 3B). Taken together, these results show that younger, as opposed to older, age post-primary DENV infections had high levels of ZIKV IgG which surged rapidly during the acute stage of disease.
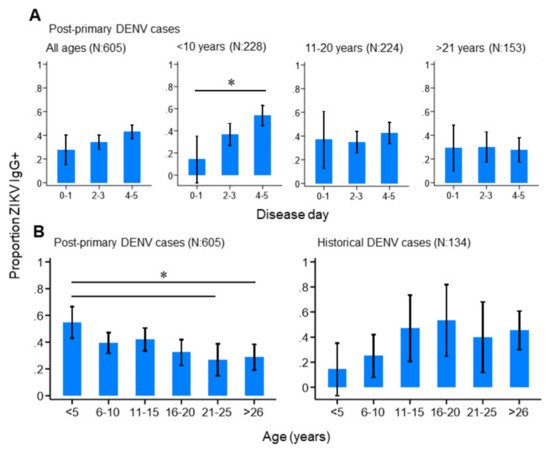

Figure 3. ZIKV IgG seroprevalence patterns among the study population. (A) ZIKV IgG seroprevalence by disease day among all, and age-stratified, post-primary dengue cases. (B) ZIKV IgG seroprevalence among post-primary and historical dengue cases. Vertical lines: 95%CI (confidence interval), (* non-overlapping 95%CI).
We then explored the spatial patterns in ZIKV and DENV IgG exposure across the Philippines in 2016 among those without active dengue infections, as ZIKV IgG is impacted by changing levels of DENV IgG during an active DENV infection (Figure 4A). We found that ZIKV historical exposure was widespread across the Philippines, with ZIKV IgG+ individuals identified in 15/17 Philippine regions. Moreover, we observed no statistical correlation between regional ZIKV and DENV IgG exposure, inferring that elevated ZIKV IgG is not attributed to higher DENV IgG (ρ: 0.26, p-value: 0.184) (Supplementary Materials Figure S3). We also found further evidence of widespread ZIKV exposure, identifying seven regions across the Philippines where DENV-negative (DENV IgG-) cases reported as ZIKV IgG-seropositive (Supplementary Materials Table S3). However, numbers were small (a total of 63 negative dengue cases in 11/17 regions across the Philippines).
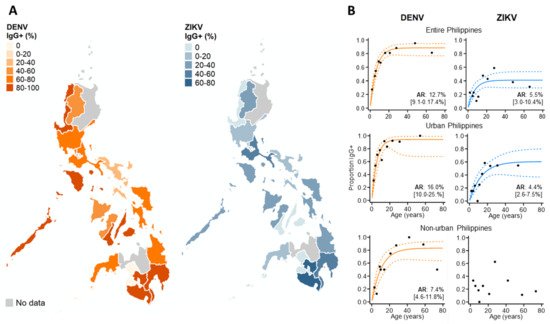

Figure 4. Immunoepidemiology of ZIKV and DENV across the Philippines during 2016. (A) Regional ZIKV/DENV IgG seroprevalence among those reporting without active DENV infections. (B) ZIKV/DENV age seroprevalence across the Philippines and stratified by urban/non-urban areas. ARs (attack rates) were calculated from the seroconversion rate (SCR) estimated among those without current DENV infections (historical and negative DENV cases) using reverse catalytic models. Black dots: observed age IgG seroprevalence. Curve: predicted age IgG seroprevalence. Dash: 95%CI.
To investigate the DENV and ZIKV annual attack rate, we used catalytic models to characterize age seroprevalence among those reporting without active DENV infections (DENV PCR- & IgM-). According to AIC, reversible, as opposed to simple catalytic models, had superior model fits. For dengue, increasing DENV IgG seroprevalence with age generated an AR estimate which suggests that 12.7% (95% CI: 9.1–17.4%) of the study population became exposed to DENV annually. DENV AR estimates were slightly higher in urban settings (AR: 16.0% (95%CI: 10.0–25.0%)) and lower in non-urban settings (7.4% (95%CI: 4.6–11.8%)). For Zika, the overall AR was lower than for dengue, with an estimated 5.5% (95%CI: 3.0–10.4%) of the study population becoming exposed annually. After stratifying by population density, the Zika FOI remained similar in urban centers across the Philippines. In contrast, we observed no increasing age ZIKV IgG seroprevalence in non-urban areas and were unable to estimate the FOI (Figure 4B).
Last, we explored whether post-primary dengue cases with/without prior ZIKV IgG exposure had a similar risk of adverse clinical/severe symptoms compared to primary DENV infections (Table 1). Adverse clinical symptoms included reporting with either warning signs of dengue or severe dengue disease. Among the 81.6% (814/997) of the study population with symptom data, 73.3% of primary DENV infections, 80.7% of post-primary DENV infections without ZIKV IgG exposure and 87.4% of post-primary DENV infections with ZIKV IgG exposure presented with adverse clinical symptoms. Moreover, compared to primary DENV infections, post-primary infections with prior Zika exposure were statistically more likely to experience adverse clinical outcomes (OR: 2.52 (95%CI: 1.42–4.49), p-value: 0.002). However, no such association was identified upon stratifying the outcome by just severe disease. Post-primary DENV infections with prior exposure to Zika did not have a significantly higher risk of presenting with severe disease compared to primary infections (OR: 1.31 (95% CI: 0.66–2.60), p-value: 0.438). This pattern is likely attributed to severe dengue being a rare disease outcome. It should also be noted that, among the study population with active disease, those with elevated DENV IgG, on average, had higher ZIKV IgG. Moreover, those with active disease and elevated DENV IgG were more likely to present with adverse clinical symptoms (Supplementary Materials Figure S4).
Table 1. Clinical manifestations associated with primary DENV infections (ZIKV IgG-), post-primary DENV infections (ZIKV IgG-) and post-primary DENV infections (ZIKV IgG+). Adverse clinical symptoms: dengue warning signs or severe symptoms. OR: odds ratios.
| Reported DENV/ZIKV | N | Adverse Clinical Symptoms | Severe Symptoms | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Immune Status | % | OR | [95% CI] | p | -Value | % | OR | [95% CI] | p | -Value | |
| Primary DENV | 131 | 73.3 | 1 (ref) | 9.2 | 1 (ref) | ||||
| (ZIKV IgG-) | |||||||||
| Post-primary DENV | 306 | 80.7 | 1.53 | [0.94–2.47] | 0.084 | 6.9 | 0.69 | [0.33–1.34] | 0.254 |
| (ZIKV IgG-) | |||||||||
| Post-primary DENV | 190 | 87.4 | 2.52 | [1.42–4.49] | 0.002 | 12.6 | 1.31 | [0.66–2.60] | 0.438 |
| (ZIKV IgG+) |
References
- World Health Organization. Zika Epidemiological Update Report. Available online: https://www.who.int/emergencies/diseases/zika/zika-epidemiology-update-july-2019.pdf (accessed on 14 July 2021).
- Lowe, R.; Barcellos, C.; Brasil, P.; Cruz, O.G.; Honório, N.A.; Kuper, H.; Carvalho, M.S. The Zika Virus Epidemic in Brazil: From Discovery to Future Implications. Int. J. Environ. Res. Public Health 2018, 15, 96.
- Cao-Lormeau, V.-M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539.
- Brady, O.J.; Osgood-Zimmerman, A.; Kassebaum, N.J.; Ray, S.E.; de Araújo, V.E.M.; Da Nóbrega, A.A.; Frutuoso, L.C.V.; Lecca, R.C.R.; Stevens, A.; Zoca de Oliveira, B.; et al. The association between Zika virus infection and microcephaly in Brazil 2015-2017: An observational analysis of over 4 million births. PLoS Med. 2019, 16, e1002755.
- Lessler, J.; Chaisson, L.H.; Kucirka, L.M.; Bi, Q.; Grantz, K.; Salje, H.; Carcelen, A.C.; Ott, C.T.; Sheffield, J.S.; Ferguson, N.M.; et al. Assessing the global threat from Zika virus. Science 2016, 353, aaf8160.
- Ruchusatsawat, K.; Wongjaroen, P.; Posanacharoen, A.; Rodriguez-Barraquer, I.; Sangkitporn, S.; Cummings, D.A.T.; Salje, H. Long-term circulation of Zika virus in Thailand: An observational study. Lancet Infect. Dis. 2019, 19, 439–446.
- Rodriguez-Barraquer, I.; Costa, F.; Nascimento, E.J.M.; Nery, N.; Castanha, P.M.S.; Sacramento, G.A.; Cruz, J.; Carvalho, M.; De Olivera, D.; Hagan, J.E.; et al. Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. Science 2019, 363, 607–610.
- Ng, D.H.L.; Ho, H.J.; Chow, A.; Wong, J.; Kyaw, W.M.; Tan, A.; Chia, P.Y.; Choy, C.Y.; Tan, G.; Yeo, T.W.; et al. Correlation of clinical illness with viremia in Zika virus disease during an outbreak in Singapore. BMC Infect. Dis. 2018, 18, 1–7.
- Okafor, I.I. Zika Virus: The Emerging Global Health Challenge. Divers. Equal. Health Care 2016, 13.
- Stone, M.; Bakkour, S.; Lanteri, M.C.; Brambilla, D.; Simmons, G.; Bruhn, R.; Kaidarova, Z.; Lee, T.H.; Orlando Alsina, J.; Williamson, P.C.; et al. Zika virus RNA and IgM persistence in blood compartments and body fluids: A prospective observational study. Lancet Infect. Dis. 2020, 20, 1446–1456.
- Pasquier, C.; Joguet, G.; Mengelle, C.; Chapuy-Regaud, S.; Pavili, L.; Prisant, N.; Izopet, J.; Bujan, L.; Mansuy, J.M. Kinetics of anti-ZIKV antibodies after Zika infection using two commercial enzyme-linked immunoassays. Diagn. Microbiol. Infect. Dis. 2018, 90, 26–30.
- St John, A.L.; Rathore, A.P.S. Adaptive immune responses to primary and secondary dengue virus infections. Nat. Rev. Immunol. 2019, 19, 218–230.
- Halstead, S.B. Dengue Antibody-Dependent Enhancement: Knowns and Unknowns. Microbiol. Spectr. 2014, 2.
- Martín-Acebes, M.A.; Saiz, J.-C.; Jiménez de Oya, N. Antibody-Dependent Enhancement and Zika: Real Threat or Phantom Menace? Front. Cell. Infect. Microbiol. 2018, 8, 44.
- Culshaw, A.; Mongkolsapaya, J.; Screaton, G.R. The immunopathology of dengue and Zika virus infections. Curr. Opin. Immunol. 2017, 48, 1–6.
- Katzelnick, L.C.; Narvaez, C.; Arguello, S.; Lopez Mercado, B.; Collado, D.; Ampie, O.; Elizondo, D.; Miranda, T.; Bustos Carillo, F.; Mercado, J.C.; et al. Zika virus infection enhances future risk of severe dengue disease. Science 2020, 369, 1123–1128.
- George, J.; Valiant, W.G.; Mattapallil, M.J.; Walker, M.; Huang, Y.-J.S.; Vanlandingham, D.L.; Misamore, J.; Greenhouse, J.; Weiss, D.E.; Verthelyi, D.; et al. Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques. Sci. Rep. 2017, 7, 1–10.
- Ariën, K.K.; Michiels, J.; Foqué, N.; Heyndrickx, L.; Van Esbroeck, M. Can Zika virus antibodies cross-protect against dengue virus? Lancet Glob. Health 2018, 6, e494.
- Valiant, W.G.; Huang, Y.-J.S.; Vanlandingham, D.L.; Higgs, S.; Lewis, M.G.; Mattapallil, J.J. Zika convalescent macaques display delayed induction of anamnestic cross-neutralizing antibody responses after dengue infection. Emerg. Microbes Infect. 2018, 7, 130.
- Elisa, N.S. Zika Virus Infections EUROIMMUN Test Systems for the Diagnosis of Zika Virus Infections. Available online: https//:www.euroimmun.com/documents/Indications/Infections/Zika-virus/HI_2668_I_UK_B.pdf (accessed on 14 July 2021).
- Huzly, D.; Hanselmann, I.; Schmidt-Chanasit, J.; Panning, M. High specificity of a novel Zika virus ELISA in European patients after exposure to different flaviviruses. Eurosurveillance 2016, 21.
- Steinhagen, K.; Probst, C.; Radzimski, C.; Schmidt-Chanasit, J.; Emmerich, P.; Van Esbroeck, M.; Schinkel, J.; Grobusch, M.P.; Goorhuis, A.; Warnecke, J.M.; et al. Serodiagnosis of Zika virus (ZIKV) infections by a novel NS1-based ELISA devoid of cross-reactivity with dengue virus antibodies: A multicohort study of assay performance, 2015 to 2016. Eurosurveillance 2016, 21, 30426.
- Medialdea-Carrera, R.; Levy, F.; Castanha, P.; de Sequeira, P.C.; Brasil, P.; Lewis-Ximenez, L.L.; Turtle, L.; Solomon, T.; Bispo de Filippis, A.M.; Brown, D.W.; et al. A systematic evaluation of IgM and IgG antibody assay accuracy in diagnosing acute Zika Virus infection in Brazil; lessons relevant to emerging infections. bioRxiv 2020.
- Kikuti, M.; Tauro, L.B.; Moreira, P.S.S.; Campos, G.S.; Paploski, I.A.D.; Weaver, S.C.; Reis, M.G.; Kitron, U.; Ribeiro, G.S. Diagnostic performance of commercial IgM and IgG enzyme-linked immunoassays (ELISAs) for diagnosis of Zika virus infection. Virol. J. 2018, 15, 108.
- Morales, I.; Rosenberger, K.D.; Magalhaes, T.; Morais, C.N.L.; Braga, C.; Marques, E.T.A.; Calvet, G.A.; Damasceno, L.; Brasil, P.; Bispo de Filippis, A.M.; et al. Diagnostic performance of anti-Zika virus IgM, IgAM and IgG ELISAs during co-circulation of Zika, dengue, and chikungunya viruses in Brazil and Venezuela. PLoS Negl. Trop. Dis. 2021, 15, e0009336.
- Liao, T.; Wang, X.; Donolato, M.; Harris, E.; Cruz, M.M.; Balmaseda, A.; Wang, R.Y.L. Evaluation of ViroTrack Sero Zika IgG/IgM, a New Rapid and Quantitative Zika Serological Diagnostic Assay. Diagnostics 2020, 10, 372.
- Buerano, C.C.; Pangilinan, L.A.S.; Dimamay, M.T.A.; Mapua, C.A.; Dimamay, M.P.S.; Matias, R.R.; Natividad, F.F.; Daroy, M.L.G.; Hasebe, F.; Morita, K.; et al. Zika virus infection, philippines, 2012. Emerg. Infect. Dis. 2020, 26, 2300–2301.
- Alera, M.T.; Hermann, L.; Tac-An, I.A.; Klungthong, C.; Rutvisuttinunt, W.; Manasatienkij, W.; Villa, D.; Thaisomboonsuk, B.; Velasco, J.M.; Chinnawirotpisan, P.; et al. Zika virus infection, philippines, 2012. Emerg. Infect. Dis. 2015, 21, 722–724.
- Lonogan, K.; de Guzman, A.; Delos Reyes, V.C.; Sucaldito, M.N.; Avelino, F. The enhanced Zika surveillance in the Philippines, November 14, 2016–February 28, 2017. Int. J. Infect. Dis. 2020, 101, 232–233.
More
