The primary function of the endothelial cells (EC) lining the inner surface of all vessels is to regulate permeability of vascular walls and to control exchange between circulating blood and tissue fluids of organs. The EC actin cytoskeleton plays a crucial role in maintaining endothelial barrier function. Actin cytoskeleton reorganization result in EC contraction and provides a structural basis for the increase in vascular permeability, which is typical for many diseases. Actin cytoskeleton in non-muscle cells presented two actin isoforms: non-muscle β-cytoplasmic and γ-cytoplasmic actins (β-actins and γ-actins), which are encoded by ACTB and ACTG1 genes, respectively. They are ubiquitously expressed in the different cells in vivo and in vitro and the β/γ-actin ratio depends on the cell type. Both cytoplasmic actins are essential for cell survival, but they perform various functions in the interphase and cell division and play different roles in neoplastic transformation.
- endothelial cell
- endothelial barrier function
- cytoskeleton
- non-muscle actin isoforms
- β-actin
- γ-actin
1. Introduction
2. Cytoplasmic Actin Isoforms: Structures and Functions in the Interphase Non-Muscle Cells
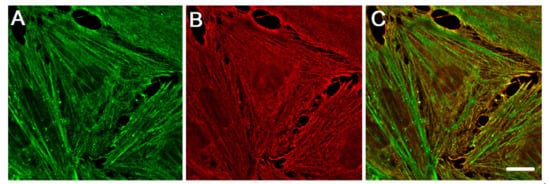
The family of actin proteins is highly conserved. The greatest difference is observed in the amino acid sequence of muscle and non-muscle isoforms [2]. Two cytoplasmic actins and many actin-binding proteins, which provide the organization of various structures, form the microfilament cytoskeleton of non-muscle cells. The amino acid sequences of cytoplasmic β-actin and γ-actin (hereafter β-actin and γ-actin) differ only in four residues located at the N-terminus of the polypeptide chain [3]. β-Actin gene is essential for survival during embryonic development of mammals. Embryos of mice lacking β-actin are much smaller in size and die at the early stages of development [7]. Embryos without γ-actin pass the prenatal period of development with some delay and they experience an increased mortality rate after birth [8]. Mouse embryonic fibroblasts with β-actin knockout show reduced motility compared to normal cells [7]. There is a pronounced compensatory expression of α-smooth muscle actin and activation of the Rho signaling pathway in these fibroblasts. ROCK inhibitors restored motility of cells without β-actin. γ-Actin is an important structural element and positive regulator of cell migration. Knockdown of γ-actin results in excessive phosphorylation of cofilin and myosin light chain, which indicates ROCK activation, increased contractility, and inhibition of the cell motility [9,10,11,12][9][10][11][12]. Morphological studies of the structures that are formed by non-muscle actins became possible due to highly specific monoclonal antibodies against β-actin and γ-actin and the method of confocal microscopy [9]. In the non-muscle cells of different origin (epithelial, endothelial, and fibroblasts) β-actin and γ-actin organize different cytoskeletal structures that are diversely located within the cell, and can perform distinct functions [9,10,13][9][10][13]. In fibroblasts, β-actin is predominantly located in the stress fibers and in the focal contacts area; cortical and lamellar branched actin network consists of γ-actin (Figure 1A,B). In the lamellipodia, the colocalization of β- and γ-actin is observed (Figure 1B). β-Actin filaments are involved in the processes of cell contraction. In the epithelial cells, β-actin forms basal microfilament bundles and participates in the adhesion junctions; γ-actin organizes the cortical (dorsal) network of actin filaments (Figure 1D) and some stress fibers [9].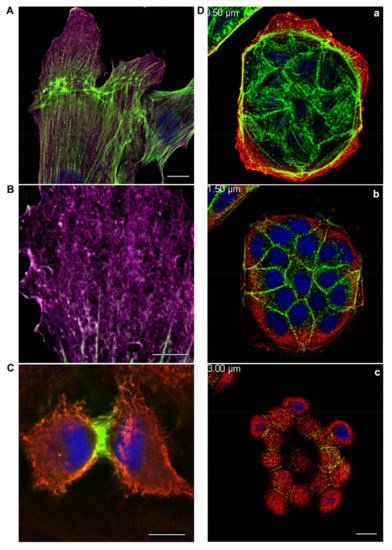
3. Different Impact of Actin Isoforms on the Process of Cell Division
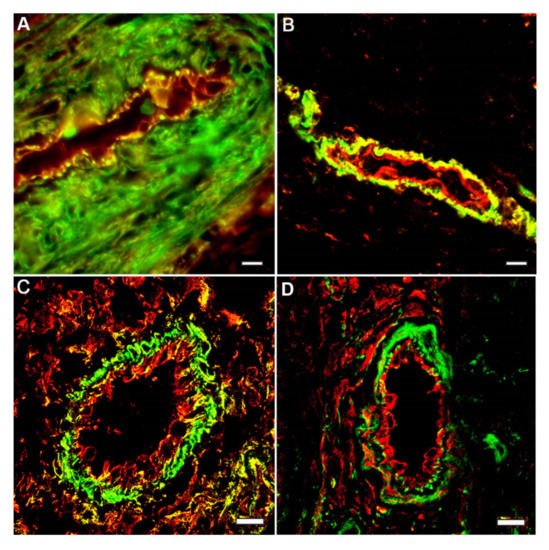
4. Crucial Involvement of Cytoplasmic Actins in the Endothelial Cell Motility and Angiogenesis
References
- Perrin, B.J.; Ervasti, J.M. The actin gene family: Function follows isoform. Cytoskeleton 2010, 67, 630–634.
- Ampe, C.; Van Troys, M. Mammalian Actins: Isoform-Specific Functions and Diseases. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2016.
- Vandekerckhove, J.; Weber, K. At least six different actins are expressed in a higher mammal: An analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J. Mol. Biol. 1978, 126, 783–802.
- Vandekerckhove, J.; Leavitt, J.; Kakunaga, T.; Weber, K. Coexpression of a mutant beta-actin and the two normal beta- and gamma-cytoplasmic actins in a stably transformed human cell line. Cell 1980, 22, 893–899.
- Otey, C.A.; Kalnoski, M.H.; Bulinski, J.C. Identification and quantification of actin isoforms in vertebrate cells and tissues. J. Cell. Biochem. 1987, 34, 113–124.
- Sheterline, P.; Clayton, J.; Sparrow, J. Actin. Protein Profile 1995, 2, 1–103.
- Bunnell, T.M.; Burbach, B.J.; Shimizu, Y.; Ervasti, J.M. β-Actin specifically controls cell growth, migration, and the G-actin pool. Mol. Biol. Cell 2011, 22, 4047–4058.
- Bunnell, T.M.; Ervasti, J.M. Delayed embryonic development and impaired cell growth and survival in Actg1 null mice. Cytoskeleton 2010, 67, 564–572.
- Dugina, V.; Zwaenepoel, I.; Gabbiani, G.; Clement, S.; Chaponnier, C.; Clément, S.; Chaponnier, C. Beta and gamma-cytoplasmic actins display distinct distribution and functional diversity. J. Cell Sci. 2009, 122, 2980–2988.
- Lechuga, S.; Baranwal, S.; Li, C.; Naydenov, N.G.; Kuemmerle, J.F.; Dugina, V.; Chaponnier, C.; Ivanov, A.I. Loss of γ-cytoplasmic actin triggers myofibroblast transition of human epithelial cells. Mol. Biol. Cell 2014, 25, 3133–3146.
- Shum, M.S.Y.; Pasquier, E.; Po’uha, S.T.; O’Neill, G.M.; Chaponnier, C.; Gunning, P.W.; Kavallaris, M. γ-Actin regulates cell migration and modulates the ROCK signaling pathway. FASEB J. 2011, 25, 4423–4433.
- Malek, N.; Mrówczyńska, E.; Michrowska, A.; Mazurkiewicz, E.; Pavlyk, I.; Mazur, A.J. Knockout of ACTB and ACTG1 with CRISPR/Cas9(D10A) technique shows that non-muscle β and γ actin are not equal in relation to human melanoma cells’ motility and focal adhesion formation. Int. J. Mol. Sci. 2020, 21, 2746.
- Baranwal, S.; Naydenov, N.G.; Harris, G.; Dugina, V.; Morgan, K.G.; Chaponnier, C.; Ivanov, A.I. Nonredundant roles of cytoplasmic β- and γ-actin isoforms in regulation of epithelial apical junctions. Mol. Biol. Cell 2012, 23, 3542–3553.
- Latham, S.L.; Chaponnier, C.; Dugina, V.; Couraud, P.; Grau, G.E.R.; Combes, V. Cooperation between β- and γ-cytoplasmic actins in the mechanical regulation of endothelial microparticle formation. FASEB J. 2013, 27, 672–683.
- Shakhov, A.S.; Verin, A.D.; Alieva, I.B. Reorganization of endothelial cells cytoskeleton during formation of functional monolayer in vitro. Cell Tissue Biol. 2014, 8, 138–151.
- Shakhov, A.S.; Dugina, V.B.; Alieva, I.B. Reorganization of actin and microtubule systems in human vein endothelial cells during intercellular contact formation. Cell Tissue Biol. 2015, 9, 299–309.
- Shakhov, A.S.; Dugina, V.B.; Alieva, I.B. Structural Features of Actin Cytoskeleton Required for Endotheliocyte Barrier Function. Biochemistry 2019, 84, 358–369.
- Piccin, A.; Murphy, W.G.; Smith, O.P. Circulating microparticles: Pathophysiology and clinical implications. Blood Rev. 2007, 21, 157–171.
- George, F.D. Microparticles in vascular diseases. Thromb. Res. 2008, 122, S55–S59.
- Pasquier, E.; Tuset, M.-P.; Sinnappan, S.; Carnell, M.; Macmillan, A.; Kavallaris, M. γ-Actin plays a key role in endothelial cell motility and neovessel maintenance. Vasc. Cell 2015, 7, 2.
- Alieva, I.B.; Zemskov, E.A.; Smurova, K.M.; Kaverina, I.N.; Verin, A.D. The leading role of microtubules in endothelial barrier dysfunction: Disassembly of peripheral microtubules leaves behind the cytoskeletal reorganization. J. Cell. Biochem. 2013, 114, 2258–2272.
- Small, J.V.; Kaverina, I. Microtubules meet substrate adhesions to arrange cell polarity. Curr. Opin. Cell Biol. 2003, 15, 40–47.
- Preciado López, M.; Huber, F.; Grigoriev, I.; Steinmetz, M.O.; Akhmanova, A.; Dogterom, M.; Koenderink, G.H. In vitro reconstitution of dynamic microtubules interacting with actin filament networks. In Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 2014; Volume 540, pp. 301–320. ISBN 9780123979247.
- Po‘uha, S.T.; Kavallaris, M. Gamma-actin is involved in regulating centrosome function and mitotic progression in cancer cells. Cell Cycle 2015, 14, 3908–3919.
- Dugina, V.; Alieva, I.; Khromova, N.; Kireev, I.; Gunning, P.W.; Kopnin, P. Interaction of microtubules with the actin cytoskeleton via cross-talk of EB1-containing +TIPs and γ-actin in epithelial cells. Oncotarget 2016, 7, 72699–72715.
- Shagieva, G.S.; Alieva, I.B.; Chaponnier, C.; Dugina, V.B. Divergent Impact of Actin Isoforms on Division of Epithelial Cells. Biochemistry 2020, 85, 1072–1081.
- Chen, A.; Ulloa Severino, L.; Panagiotou, T.C.; Moraes, T.F.; Yuen, D.A.; Lavoie, B.D.; Wilde, A. Inhibition of polar actin assembly by astral microtubules is required for cytokinesis. Nat. Commun. 2021, 12, 2409.
- Dugina, V.; Khromova, N.; Rybko, V.; Blizniukov, O.; Shagieva, G.; Chaponnier, C.; Kopnin, B.; Kopnin, P. Tumor promotion by γ and suppression by β non-muscle actin isoforms. Oncotarget 2015, 6, 14556–14571.
- Dugina, V.; Shagieva, G.; Khromova, N.; Kopnin, P. Divergent impact of actin isoforms on cell cycle regulation. Cell Cycle 2018, 17, 2610–2621.
- Zhang, L.J.; Tao, B.B.; Wang, M.J.; Jin, H.M.; Zhu, Y.C. PI3K p110α Isoform-Dependent Rho GTPase Rac1 Activation Mediates H2S-Promoted Endothelial Cell Migration via Actin Cytoskeleton Reorganization. PLoS ONE 2012, 7, e44590.
