Cardiovascular diseases such as coronary artery occlusion, hypertensive heart disease and stroke, high blood pressure, myocardial infarction, cerebrovascular disease, heart failure, rheumatic heart disease, congenital heart disease and cardiomyopathies generate thousands of patients with high mortality and morbidity worldwide. The appearance of these diseases increases with the aging of the population. In addition, these diseases are complicated by some comorbidity that patients present (overweight, obesity, diabetes mellitus, etc.). The installation and development of these diseases are closely linked to metabolic changes that generate a state of oxidative stress, due to the excessive production of reactive oxygen species (ROS) and reactive nitrogen species (RNS).
- cardiovascular diseases
- glutathione
- reactive oxygen species
- reactive nitrogen species
- oxidative stress
1. General Information
Cardiovascular diseases (CVD) (such as coronary artery occlusion, hypertensive heart disease and stroke) are diseases that generate thousands of patients with a high mortality rate worldwide. Many of these cardiovascular pathologies, during their development, generate a state of oxidative stress that leads to a deterioration of the patient's conditions associated with the generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS). Within these reactive species we find superoxide anion (O2•-), hydroxyl radical (•OH), nitric oxide (NO•), as well as other species of non-free radicals such as hydrogen peroxide (H2O2), acid hypochlorous (HClO) and peroxynitrite (ONOO-). A molecule that actively participates in counteracting the oxidizing effect of reactive species is reduced glutathione (GSH), a tripeptide that is present in all tissues and that its synthesis and / or regeneration is very important to be able to respond to the increase in oxidizing agents. . In this review, we will address the role of glutathione, its synthesis in both the heart and liver, and its importance in preventing or reducing the deleterious effects of ROS in cardiovascular disease.
2. Cardiovascular diseases
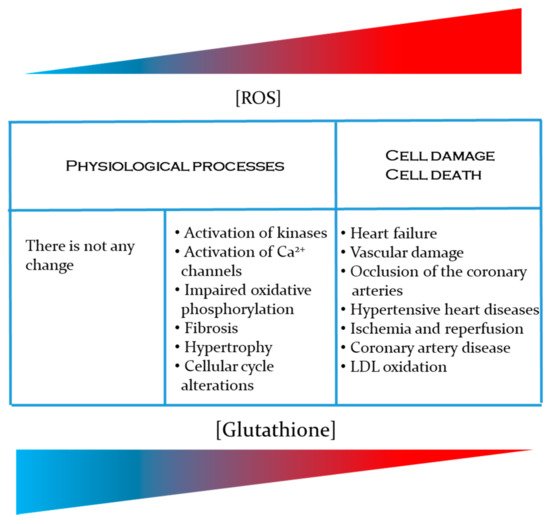
Cardiovascular diseases such as coronary artery occlusion, hypertensive heart disease and stroke, high blood pressure, myocardial infarction, cerebrovascular disease, heart failure, rheumatic heart disease, congenital heart disease and cardiomyopathies generate thousands of patients with high mortality and morbidity worldwide [ 1 , 2 , 3 , 4 ]. The occurrence of these diseases increases with the aging of the population [ 5 , 6]. In addition, these diseases are complicated by some comorbidity that patients present (overweight, obesity, diabetes mellitus, etc.). The installation and development of these diseases are closely linked to metabolic changes that generate a state of oxidative stress, due to the excessive production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) [ 7 , 8 ]. The deficiency of antioxidant molecules in elderly humans has been shown to be due to a significant reduction in their synthesis. A diet enriched with cysteine and glycine, the precursors of glutathione, fully restores cellular glutathione synthesis and GSH concentration, reduces levels of oxidative stress, and thus prevents damage associated with oxidative stress and aging [ 9]. In the case of cardiovascular pathologies, the oxidative stress state leads to a deterioration of the patient's conditions associated with the generation of ROS and RNS. Superoxide anion (O2•-), hydroxyl radical (•OH), nitric oxide (NO•) and other species of non-free radicals such as hydrogen peroxide (H2O2), hypochlorous acid (HClO) and peroxynitrite (ONOO-) are all found within these reactive species [ 10 ]. NADPH oxidase (NOX), lipoxygenase, cyclooxygenase (COX), xanthine oxidase (XO), uncoupled nitric oxide synthases (NOS), cytochrome P450, and mitochondrial respiration are the most common enzymatic causes of ROS in CVD [11]. A molecule that is actively involved in counteracting the oxidizing effect of reactive species is reduced glutathione (GSH). In the present review, we describe the role that glutathione plays in preventing an increase in ROS and RNS responsible for the development of cardiovascular diseases. Specifically, the following aspects are described: (i) description of ROS and RNS and antioxidant defenses, (ii) the importance of reduced glutathione (GSH) as an antioxidant agent, (iii) the participation of GSH in the prevention of cardiovascular diseases, (iv) the relationship between the production of reactive species and the development of cardiovascular diseases. Since several cardiovascular diseases are closely related to an increase in reactive oxygen and nitrogen species.
3 Conclusions
Many diseases are due to an imbalance between the rate of oxidant production (free radicals or reactive species) and the activity of cellular antioxidant systems. Glutathione plays a central role as an antioxidant defense, as well as in the regulation of the pathways involved in cellular homeostasis, not only as a detoxifier of endogenous and exogenous compounds, but also through its participation in processes related to cell proliferation, apoptosis, gene expression, regulation of the immune system and metabolism of cellular compounds, among others. Alterations in GSH homeostasis are related to the etiology and / or progression of a variety of diseases in addition to the listed CVD.
References
- Bachhawat, A.K.; Yadav, S.; Jainarayanan, A.K.; Dubey, P. Heart failure and the glutathione cycle: An integrated view. Biochem. J. 2020, 477, 3123–3130.
- Ballatori, N.; Krance, S.M.; Marchan, R.; Hammond, C.L. Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol. Asp. Med. 2009, 30, 13–28.
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009, 390, 191–214.
- Higashikuni, Y.; Sainz, J.; Nakamura, K.; Takaoka, M.; Enomoto, S.; Iwata, H.; Tanaka, K.; Sahara, M.; Hirata, Y.; Nagai, R.; et al. The ATP-Binding Cassette Transporter ABCG2 Protects Against Pressure Overload–Induced Cardiac Hypertrophy and Heart Failure by Promoting Angiogenesis and Antioxidant Response. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 654–661.
- Dai, D.-F.; Chiao, Y.A.; Marcinek, D.J.; Szeto, H.H.; Rabinovitch, P.S. Mitochondrial oxidative stress in aging and healthspan. Longev. Health 2014, 3, 6.
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162.
- Andreadou, I.; Schulz, R.; Papapetropoulos, A.; Turan, B.; Ytrehus, K.; Ferdinandy, P.; Daiber, A.; Di Lisa, F. The role of mitochondrial reactive oxygen species, NO and H2S in ischaemia/reperfusion injury and cardioprotection. J. Cell. Mol. Med. 2020, 24, 6510–6522.
- Griendling, K.; Touyz, R.M.; Zweier, J.L.; Dikalov, S.; Chilian, W.; Chen, Y.-R.; Harrison, D.G.; Bhatnagar, A. Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System. Circ. Res. 2016, 119, e39–e75.
- Sekhar, R.V.; Patel, S.G.; Guthikonda, A.P.; Reid, M.; Balasubramanyam, A.; Taffet, G.E.; Jahoor, F. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am. J. Clin. Nutr. 2011, 94, 847–853.
- Moris, D.; Spartalis, M.; Spartalis, E.; Karachaliou, G.-S.; Karaolanis, G.I.; Tsourouflis, G.; Tsilimigras, D.I.; Tzatzaki, E.; Theocharis, S. The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. Ann. Transl. Med. 2017, 5, 326.
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Adv. Med. 2016, 2016, 9152732.
- Gould, R.; Zhou, Y.; Yakaitis, C.L.; Love, K.; Reeves, J.; Kong, W.; Coe, E.; Xiao, Y.; Pazdro, R. Heritability of the aged glutathione phenotype is dependent on tissue of origin. Mamm. Genome 2018, 29, 619–631.
- García-Giménez, J.L.; Romá-Mateo, C.; Pérez-Machado, G.; Peiró-Chova, L.; Pallardó, F.V. Role of glutathione in the regulation of epigenetic mechanisms in disease. Free Radic. Biol. Med. 2017, 112, 36–48.
- Forman, H.J. Glutathione—From antioxidant to post-translational modifier. Arch. Biochem. Biophys. 2016, 595, 64–67.
- Zhao, Z.; Hou, Y.; Zhou, W.; Keerthiga, R.; Fu, A. Mitochondrial transplantation therapy inhibit carbon tetrachloride-induced liver injury through scavenging free radicals and protecting hepatocytes. Bioeng. Transl. Med. 2020, 6, e10209.
- Cowan, D.B.; Yao, R.; Akurathi, V.; Snay, E.R.; Thedsanamoorthy, J.K.; Zurakowski, D.; Ericsson, M.; Friehs, I.; Wu, Y.; Levitsky, S.; et al. Intracoronary Delivery of Mitochondria to the Ischemic Heart for Cardioprotection. PLoS ONE 2016, 11, e0160889.
