The heavy metal cadmium (Cd), as one of the major environmentally toxic pollutants, has serious impacts on the growth, development, and physiological functions of plants and animals, leading to deterioration of environmental quality and threats to human health.
- heavy metals
- cadmium pollution
- phytoremediation
- enrichment mechanisms
1. Introduction
With the acceleration of modern industrialisation, the problem of heavy metal pollution has become increasingly prominent [1,2,3][1][2][3]. At present, nearly 20 million hectares of farmland in China are polluted by heavy metals such as mercury, cadmium (Cd), and lead [4]. In 2015, a soil research survey showed that 16.1% of China’s soil and 19.4% of agricultural soil were polluted by heavy metals, of which cadmium pollution (7.0%) was the most serious. The Ministry of Ecology and Environment of China announced in 2019 that cadmium was still the main heavy metal pollutant in the soil [5,6,7][5][6][7].
Cd is a toxic heavy metal that can exist in soil, water, and the atmosphere in various forms [8,9][8][9]. Cd0in the atmosphere can become immobilised by combining with iron (Fe) and manganese (Mn) oxides and can also be atmospherically deposited on rain, dust, and snow [10]. Cd in soil and water is usually in an exchangeable state (CdCl2and other water-soluble forms), carbonate-bound state (such as CdHCO3−), organic-bound state (combined with organic matter in the environment), or residual state (such as H2SiO3) [8].
The biomass, root length, plant height, and the chlorophyll ofCeratopteris pteridoideswere reduced when exposed to water containing 20 μM Cd [16][11]. The length and dry weight of marigold were reduced when grown in soil containing 50 mg kg−1(DW) Cd from the soil is taken up by crop plants through a migration process and enters the food chain [18,19][12][13]. Cadmium can also cause damage to the liver and brain, leading to high blood pressure and even cancer [14,20,21,22,23][14][15][16][17][18].
As an abiotic stress factor, Cd can affect the growth and development of plants to varying degrees [14]. When Cd in the environment exceeds a certain concentration, it will stimulate oxidative stress in plants, induce lipid peroxidation, and increase the accumulation of reactive oxygen species (ROS), leading to oxidative damage [20,24,25,26][15][19][20][21]. In addition, Cd can also cause slower plant growth, decreased chlorophyll content, yellow leaves, and slower photosynthetic rate. Therefore, the growth, development, and physiological and biochemical effects of plants are affected, and high Cd concentration can even cause plant death [16,27,28][11][22][23].
Among them, hyperaccumulators can over-absorb heavy metals and transport and retain them in the shoots [30,31,32][24][25][26]. Plant species that contain more than 1000 μg of heavy metal per gram (DW) are called hyperaccumulators [26][21]. Compared with normal plants, hyperaccumulators can not only maintain normal physiological function in a high-concentration heavy metal environment but can also absorb heavy metals to enrich them [12,38][27][28]. Therefore, hyperaccumulators show great potential in repairing Cd pollution and have become a research hotspot in the field of heavy metal pollution.
2. Uptake, Transport, and Distribution of Cd in Plants
First, Cd interacts electrostatically with plant root secretions and negatively charged carboxyl groups on the root cell wall, thereby adsorbing onto plant roots [48,49][29][30]. Studies have shown that, as the Cd concentration in the environment increases, the Cd content in plants also increases, but the rate of Cd transport from plant roots to shoots decreases [50][31]. the environment is absorbed into plants through competition with metal cations such as Cu2+, Fe2+, In addition, the content of heavy metal particles in the air in polluted areas is significantly related to the content of heavy metals in plant leaves, which is speculated to be related to atmospheric deposition [54,55][32][33].
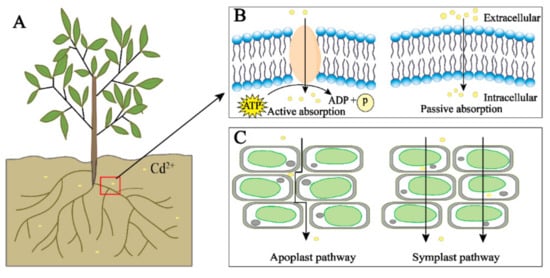
There are two main transport pathways of Cd in plants: the symplast and apoplast pathways. The apoplast pathway is where Cd is transported through the gap between plant cell walls and the gap between the cell wall and the plasma membrane; the symplast pathway is where Cd is transported into the cell through the transporters and then transported between the cells through the plasmodesmata (Figure 1C) [56][34]. Cd in the environment is actively or passively absorbed by plants, first loaded in the root xylem, and then further transported through the xylem to the plant shoots [58,59][35][36]. In plants, Cd can also be efficiently transported from senescent tissues to young tissues through the phloem to facilitate the transport and redistribution of Cd in plants [30][24].
Plants require the participation of transporters in the process of uptake and transport of Cd, which mainly include yellow-stripe 1-like transporter (YSL) The YSL protein (YS1) of corn’s main function is to transport the Fe–phytosiderophore complex needed for corn growth into corn root epidermal cells [71,72][37][38]. andBjYSL6.1, which was specifically upregulated in shoots after Cd treatment, indicating that the YSL protein is involved in the transport of Cd from roots to shoots. Although many studies have shown that the YSL protein is involved in the transport of Cd, there are few studies on YSL protein as a whole.
The ZIP family is responsible for the uptake and transport of essential and nonessential metal ions in plants and participates in the uptake and transport of a variety of divalent cations, including Cd2+[62,77][39][40]. (zrt1andzrt2mutants were inhibited in zinc uptake due to lack of high-and low-affinity zinc uptake systems), and the uptake of zinc by yeast was increased after the expression ofZIP1andZIP3genes. In addition, Cd can inhibit zinc uptake mediated byZIP1, ZIP2, andZIP3, indicating that Cd can also be taken up from the soil by ZIP transporters and be transported to plants as a substrate of ZIP transporters. This is consistent with the increase in Cd accumulation afterBcZIP2expression inBrassica campestrisL. ssp.Chinensis[81][41], which fully proves that ZIP protein is involved in the process of Cd uptake and transport.
andhma2hma4mutants were more sensitive to Cd and had significantly inhibited root growth. Thehma2hma4mutant increased Cd accumulation in the roots, and the transport of Cd from roots to shoots was 2–3% of the wild type [87][42], which is consistent with the result that theAthma4mutant transport rate decreased by at least 50% [88][43]. The Cd concentration in the roots and shoots of the overexpressed riceOsHMA3strain was higher than that of the control group, and the roots showed higher Cd content at low and high Cd culture concentrations. In addition, immunostaining studies have shown that cucumberCsHMA3andCsHMA4were expressed in the tonoplast and plasma membrane of cucumber root cells, respectively [89][44], and cucumberCsHMA3andCsHMA4were expressed in the vacuole membrane and plasma membrane of cucumber root cells, respectively, indicating that HMA protein exists in the root cell membrane and tonoplast membrane and plays an important role in the uptake of Cd from roots and the transport of Cd to shoots.
NRAMP1 is the first protein discovered in the NRAMP family and participates in the process of macrophage resistance to bacterial infection by transporting Fe2+[90][45].Sedum alfredii SaNRAMP6[91][46] is expressed on the plasma membrane of epidermal cell protoplasts. This is consistent with the increase in Cd concentration in roots, stems, leaves, and whole plants [94][47] found thatHvNRAMP5was mainly expressed in root epidermal cells, and the expression of the root tip was higher than that of the root base. The presence of NRAMP protein is beneficial to the xylem parenchyma of plant roots to load Cd and transport it to young plant parts in the phloem [30][24].
In the process of heavy metal uptake and transport, the participation of transporters and their importance has received extensive attention from scholars. Many transporters, such as YSL, ZIP, HMA, and NRAMP, that participate in the uptake and transport of Cd have been studied. However, the molecular mechanism of the specific binding of the abovementioned transporter to metal ions and the relationship between the structure and function of the transporter are still unclear. These are essential in elucidating the mechanisms of Cd uptake and transport in plants.
Heavy metals such as Cd are absorbed from the environment by these transporters into plant roots and then transported to shoots, where they are distributed to various tissues of the plant. The distribution of Cd in plants varies according to plant species and varieties. The distribution of Cd in different plant tissues usually shows that the accumulation of Cd in the roots of plants is the largest, and the accumulation of Cd in shoots is less than that in roots, which is related to the retention of Cd by plant roots [88][43]. It is speculated that the Cd in these plants is mainly concentrated in the roots at low Cd concentrations.
In Wang’s study [9], the subcellular distribution (the ratio of total Cd in different subcellular structure to the total Cd in the whole cell) of Cd in soybean roots at 23 μM and 45 μM levels was as follows: cell wall (53.4–75.5%) Similar to the subcellular distribution in soybean roots, the subcellular distribution of Cd in the Cd hyperaccumulator,Solanum nigrum,was cell wall ( This indicates that the root cell wall is the main subcellular location for plants to store Cd, which is achieved by the retention effect of roots, followed by Cd transported to shoots and stored in the vacuole in the form of soluble fractions. This may be related to the fact that Cd is stored in vacuoles of plant cells through chelation and compartmentalisation, including heavy metal ions interacting with ligands.
The distribution and subcellular distribution of Cd in plants are uneven due to differences in plant species and varieties. At present, the distribution and subcellular distribution of Cd in plants as physiological and biochemical characteristics have been widely studied by scholars, but there is no unified understanding of whether the difference in its distribution is related to the significance of enrichment and tolerance.
3. The Mechanisms of Plant Enrichment of Cd
Under Cd stress, plants show a series of physiological responses to achieve detoxification and enrichment of heavy metals Current studies have shown that the main response mechanisms of plants under Cd stress are the retention effect of roots, compartmentalisation, chelation, antioxidation, stress, and osmotic adjustment [43,118][48][49]. Among them, root function, compartmentalisation, and chelation in plants are the dominant factors in the process of Cd enrichment. Other plant response mechanisms also play an important role in achieving Cd enrichment in plants, regulating physiology, and maintaining life activities.
Cd2+can stimulate the production of ROS, cause oxidative stress, and stimulate the plant’s antioxidant defence system. Antioxidant enzymes, such as superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), eliminate reactive oxygen species (ROS) through increased activity. Heat shock protein (HSP) induced by stress can upregulate antioxidant enzyme activity, upregulate MT expression, and remove denatured proteins. The increase in proline content is conducive to the elimination of ROS, and an increase in soluble protein content is conducive to maintaining cell stability and cell metabolism.
The root secretions and the cell wall of root epidermal cells play an important role in limiting Cd uptake and Cd accumulation in the roots [58][35]. Root exudates can form complexes or precipitates with external Cd, thereby retaining Cd on the root epidermis [119][50]; organic acids in different types of root exudates also have different adsorption effects on Cd in the soil, so that Cd in the soil enters the plant roots in different chemical forms. An appropriate concentration of root exudates can promote the transfer of Cd from roots to leaves for accumulation in the leaves [119][50]. Cd in roots mainly accumulates in the cortical tissues of root tips and root hairs.
After heavy metal ions enter the plant, they can be chelated or even precipitated by metallothioneins (MT), phytochelatins (PC), glutathione, oxalic acid, citric acid, and other small molecules in the plant cytoplasm and vacuoles, thus reducing their toxicity [122,123][51][52]. It is produced in response to heavy metal ions entering the plant [124,125][53][54]. The binding of heavy metal ions to the cysteine-rich regions may be one of the mechanisms that plants use for detoxification [129][55]. PC is a type of sulfhydryl polypeptide containing cysteine, glutamic acid, and glycine synthesised by plants after being stressed by heavy metals [130][56].
Plants transport heavy metals that enter the plant to inactive areas such as cell walls and vacuoles, retain them, and reduce the fluidity of heavy metals by isolating them. The transport mechanism of vacuole compartmentalisation is divided into two types: transport directly through the transporter on the vacuole, and transport through the vesicle in the cytoplasm and the vacuole membrane [136][57]. [137][58] found that Cd easily combines with amino, carboxyl, hydroxyl, and other coordination groups in cell wall proteins and polysaccharides to realise the cell wall compartmentalisation of Cd. When the Cd2+accumulation in the cell wall is higher enough, Cd is then transported to the vacuole.
Reactive oxygen species (ROS) play an important role in controlling plant growth, the abiotic stress response, system signal transmission, programmed cell death, and plant development [144][59]. When the content of ROS in plants increases, the accumulated ROS leads to membrane lipid peroxidation, cell membrane rupture, electrolyte leakage, and DNA loss, which affect the normal physiological and biochemical functions of cells [145][60]. As ROS levels increase, the antioxidant defence system in plants is activated. As an important part of the antioxidant defence system, antioxidant enzymes in plants can help eliminate ROS and reduce plant damage [26,146][21][61].
Once the plant is stressed by Cd, the plant will adjust the activity of these antioxidant enzymes accordingly, but this change will vary depending on the plant species, the length of exposure to heavy metals, and the growth stage of the plant [148][62]. This proves that the increase in antioxidant enzyme activity is directly related to the improvement of plant tolerance. Although antioxidant enzymes such as SOD, CAT, and POD can regulate their activity to eliminate ROS to protect plants within a certain concentration range, the activity of these antioxidant enzymes will still be inhibited at high Cd concentrations. Some hyperaccumulators can still maintain high enzyme activity in the presence of high concentrations of Cd (≥100 mg kg−1, DW),
It can also bind to cytoplasmic phosphorylated proteins to regulate antioxidant enzyme activity, induce melatonin production, and upregulate MT expression, thereby improving the ability of cells to tolerate Cd [157[63][64][65],158,159], which may be related to the DNA-binding domain in the heat shock transcription factor, HsfA4a [160][66]. In addition to the synthesis of HSP, plants can also induce ethylene production under Cd stress. Cd stress can upregulate the expression of 1-aminocyclopropane-1-carboxylic acid (ethylene synthesis precursor) synthase to induce ethylene synthesis, and further regulate the growth inhibition of plant roots by regulating XTH33 and LSU1 mediated by the transcription factor, EIN3 [163,164][67][68]. In addition, ethylene can control the SOD content by increasing the activity of SOD isoenzymes, but excessively high ethylene levels inhibit the activities of antioxidant enzymes such as CAT and APX [165,166][69][70].
After Cd stress, plants can also increase the cell osmotic potential by changing the content of osmotic adjustment substances, including proline, soluble protein, soluble sugar, and small molecular organic acids, thereby eliminating the toxic effect of Cd on plants [167,168][71][72]. By increasing the content of soluble protein and soluble sugar in plants, the intracellular osmotic potential can be increased, and the intracellular water content can be maintained, which is helpful in alleviating electrolyte leakage caused by oxidative stress and maintaining the normal physiological function of cells [175,176][73][74]. However, the increase in soluble protein content in soybean endosperm is accompanied by a decrease in soluble protein content and soluble sugar content in the radicle [177][75], which might be related to the inhibitory effect of Cd on the activities of hydrolytic enzymes, including α-amylase, β-amylase, acid phosphatase, and alkaline phosphatase [178][76]. This suggests that complex osmotic regulatory networks exist in plants that can regulate cell osmotic potential through a series of complex responses to Cd stress to reduce the toxic effects of Cd on plants.
4. Conclusions and Outlook
As a widespread pollutant in the environment, Cd not only affects the physiological and biochemical functions of plants but can also be ingested by the human body through the biological chain to have a serious impact on health. Cd in the environment can interact with plant root cell walls or root exudates to be adsorbed on roots, enter plant roots through active or passive absorption, be transported by symplast pathway or apoplast pathway, and transport to shoots through the xylem. In this process, Cd is combined with YSL, ZIP, HMA, NRAMP, and other transporters and is finally distributed to various tissues of the plant, mainly in the cell wall of plant roots and the vacuole of shoots. In addition, osmotic adjustment substances such as proline, soluble protein, and soluble sugar can also reduce the oxidative damage caused by Cd to plants and exert detoxification effects to further enhance the enrichment of Cd in plants.
Many scholars have obtained important research results on the molecular mechanisms of Cd uptake, distribution, and transport, as well as the molecular mechanism of plant enrichment and tolerance to Cd. However, there are still many problems that have not been resolved, such as the significance of the difference in the distribution of Cd in plants for plants to adapt to the environment and maintain their physiological functions; the chemical, morphological changes of Cd in the process of transport and the molecular mechanism of its binding to transporters; the regulatory mechanism of compartmentalisation and chelation; the regulatory mechanism of antioxidant enzyme activity under Cd stress; and the osmotic regulatory mechanism under Cd stress. In the process of Cd absorption and transport, the structure and morphology of the transporter changes, as do the interactions between different proteins. If the above problems can be solved, research on the mechanism of Cd enrichment in plants will take a big step forward, and phytoremediation technology can be used to treat heavy metal pollution.
References
- Jeong, H.; Choi, J.Y.; Lee, J.; Lim, J.; Ra, K. Heavy metal pollution by road-deposited sediments and its contribution to total suspended solids in rainfall runoff from intensive industrial areas. Environ. Pollut. 2020, 265, 115028.
- Weissmannová, H.D.; Mihočová, S.; Chovanec, P.; Pavlovský, J. Potential Ecological Risk and Human Health Risk Assessment of Heavy Metal Pollution in Industrial Affected Soils by Coal Mining and Metallurgy in Ostrava, Czech Republic. Int. J. Environ. Res. Public Health 2019, 16, 4495.
- Fang, T.; Yang, K.; Lu, W.; Cui, K.; Li, J.; Liang, Y.; Hou, G.; Zhao, X.; Li, H. An overview of heavy metal pollution in Chaohu Lake, China: Enrichment, distribution, speciation, and associated risk under natural and anthropogenic changes. Environ. Sci. Pollut. Res. 2019, 26, 29585–29596.
- Gu, J.G.; Zhou, Q.X.; Wang, X. Reused Path of Heavy Metal Pollution in Soils and its Research Advance. J. Basic Sci. Engine 2003, 02, 143–151.
- Zhao, F.-J.; Ma, Y.; Zhu, Y.-G.; Tang, Z.; McGrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2015, 49, 750–759.
- Yuan, X.; Xue, N.; Han, Z. A meta-analysis of heavy metals pollution in farmland and urban soils in China over the past 20 years. J. Environ. Sci. 2021, 101, 217–226.
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700.
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Antunes, P.M.C. Cadmium Bioavailability, Uptake, Toxicity and Detoxification in Soil-Plant System. Rev. Environ. Contam. Toxicol. 2016, 241, 73–137.
- Wang, P.; Deng, X.; Huang, Y.; Fang, X.; Zhang, J.; Wan, H.; Yang, C. Comparison of subcellular distribution and chemical forms of cadmium among four soybean cultivars at young seedlings. Environ. Sci. Pollut. Res. 2015, 22, 19584–19595.
- Pan, H.J.; Liu, J.T.; Ma, S.H.; Zhao, S.Z.; Zhong, R.; Liu, Y.B.; Zhao, J.Z. Occurrence characteristics of cadmium in atmospheric dustfall in Baotou City. J. Inner. Mong. Agric. Univ. 2010, 31, 105–109.
- Bora, M.S.; Gogoi, N.; Sarma, K.P. Tolerance mechanism of cadmium in Ceratopteris pteridoides: Translocation and subcellular distribution. Ecotoxicol. Environ. Safe 2020, 197, 110599.
- Jolly, Y.N.; Islam, A.; Akbar, S. Transfer of metals from soil to vegetables and possible health risk assessment. SpringerPlus 2013, 2, 1–8.
- Brams, E.; Anthony, W. Cadmium and lead through an agricultural food chain. Sci. Total Environ. 1983, 28, 295–306.
- Augustsson, A.; Uddh-Söderberg, T.; Filipsson, M.; Helmfrid, I.; Berglund, M.; Karlsson, H.; Hogmalm, J.; Karlsson, A.; Alriksson, S. Challenges in assessing the health risks of consuming vegetables in metal-contaminated environments. Environ. Int. 2018, 113, 269–280.
- Paithankar, J.G.; Saini, S.; Dwivedi, S.; Sharma, A.; Chowdhuri, D.K. Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction. Chemosphere 2021, 262, 128350.
- Cheng, C.-H.; Ma, H.-L.; Deng, Y.-Q.; Feng, J.; Jie, Y.-K.; Guo, Z.-X. Oxidative stress, cell cycle arrest, DNA damage and apoptosis in the mud crab (Scylla paramamosain) induced by cadmium exposure. Chemosphere 2021, 263, 128277.
- Júnior, J.E.G.P.; Moraes, P.Z.; Rodriguez, M.D.; Simões, M.R.; Cibin, F.; Pinton, S.; Junior, F.B.; Peçanha, F.M.; Vassallo, D.V.; Miguel, M.; et al. Cadmium exposure activates NADPH oxidase, renin-angiotensin system and cyclooxygenase 2 pathways in arteries, inducing hypertension and vascular damage. Toxicol. Lett. 2020, 333, 80–89.
- Satarug, S.; Moore, M.R. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ. Health Perspect. 2004, 112, 1099–1103.
- Wan, Y.; Wang, K.; Liu, Z.; Yu, Y.; Wang, Q.; Li, H. Effect of selenium on the subcellular distribution of cadmium and oxidative stress induced by cadmium in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2019, 26, 16220–16228.
- Zhang, R.R.; Zhang, P.; Du, S.T. Oxidative stress-related signals and their regulation under Cd stress: A review. J. Appl. Ecol. 2016, 27, 981–992.
- Singh, S.; Eapen, S.; D’Souza, S. Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere 2006, 62, 233–246.
- Huang, S.; Song, Q.; Li, Q.; Zhang, H.; Luo, X.; Zheng, Z. Damage of heavy metals to Vallisneria natans (V. natans) and characterization of microbial community in biofilm. Aquat. Toxicol. 2020, 225, 105515.
- Andresen, E.; Kappel, S.; Stärk, H.; Riegger, U.; Borovec, J.; Mattusch, J.; Heinz, A.; Schmelzer, C.; Matoušková, Š.; Dickinson, B.; et al. Cadmium toxicity investigated at the physiological and biophysical levels under environmentally relevant conditions using the aquatic model plant Ceratophyllum demersum. New Phytol. 2016, 210, 1244–1258.
- Hu, Y.; Tian, S.; Foyer, C.H.; Hou, D.; Wang, H.; Zhou, W.; Liu, T.; Ge, J.; Lu, L.; Lin, X. Efficient phloem transport significantly remobilizes cadmium from old to young organs in a hyperaccumulator Sedum alfredii. J. Hazard. Mater. 2019, 365, 421–429.
- Küpper, H.; Leitenmaier, B. Cadmium-accumulating plants. Met. Ions Life Sci. 2012, 11, 373–393.
- Jaffre, T.; Brooks, R.R.; Lee, J.; Reeves, R.D. Sebertia acuminata: A Hyperaccumulator of Nickel from New Caledonia. Science 1976, 193, 579–580.
- Li, G.; Li, Q.; Wang, L.; Zhang, D. Cadmium tolerance and detoxification in Myriophyllum aquaticum: Physiological responses, chemical forms, and subcellular distribution. Environ. Sci. Pollut. Res. Int. 2020, 27, 37733–37744.
- Xu, X.; Zhang, S.; Cheng, Z.; Li, T.; Jia, Y.; Wang, G.; Yang, Z.; Xian, J.; Yang, Y.; Zhou, W. Transcriptome analysis revealed cadmium accumulation mechanisms in hyperaccumulator Siegesbeckia orientalis L. Environ. Sci. Pollut. Res. 2020, 27, 18853–18865.
- Hauser, L.; Tandy, S.; Schulin, R.; Nowack, B. Column extraction of heavy metals from soils using the biodegradable chelating agent EDDS. Environ. Sci. Technol. 2005, 39, 6819–6824.
- Meychik, N.R.; Nikolaeva, Y.I.; Yermakov, I.P. Ion-exchange properties of cell walls of Spinacia oleracea L. roots under different environmental salt conditions. Biochemistry 2006, 71, 781–789.
- Palusińska, M.; Barabasz, A.; Kozak, K.; Papierniak, A.; Maślińska, K.; Antosiewicz, D.M. Zn/Cd status-dependent accumulation of Zn and Cd in root parts in tobacco is accompanied by specific expression of ZIP genes. BMC Plant Boil. 2020, 20, 37.
- De Temmerman, L.; Ruttens, A.; Waegeneers, N. Impact of atmospheric deposition of As, Cd and Pb on their concentration in carrot and celeriac. Environ. Pollut. 2012, 166, 187–195.
- Fernández, E.A.; Rossini, O.S. The composition and relationships between trace element levels in inhalable atmospheric particles (PM10) and in leaves of Nerium oleander L. and Lantana camara L/. Chemosphere 2006, 62, 1665–1672.
- Anda, A.; Illés, B.; Soós, G. Effect of cadmium pollution of atmospheric origin on field-grown maize in two consecutive years with diverse weather conditions. Acta Biol. Hung. 2013, 64, 476–489.
- Huang, X.; Duan, S.; Wu, Q.; Yu, M.; Shabala, S. Reducing Cadmium Accumulation in Plants: Structure-Function Relations and Tissue-Specific Operation of Transporters in the Spotlight. Plants 2020, 9, 223.
- Da Cunha, K.P.V.; Nascimento, C.W.A.D.; Pimentel, R.M.D.M.; Ferreira, C.P. Cellular localization of cadmium and structural changes in maize plants grown on a cadmium contaminated soil with and without liming. J. Hazard. Mater. 2008, 160, 228–234.
- Schaaf, G.; Ludewig, U.; Erenoglu, B.E.; Mori, S.; Kitahara, T.; von Wirén, N. ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. J. Biol. Chem. 2004, 279, 9091–9096.
- Murata, Y.; Ma, J.F.; Yamaji, N.; Ueno, D.; Nomoto, K.; Iwashita, T. A specific transporter for iron (III)-phytosiderophore in barley roots. Plant J. 2006, 46, 563–572.
- Guerinot, M.L. The ZIP family of metal transporters. Biochim. Biophys. Acta Biomembr. 2000, 1465, 190–198.
- Palmer, C.M.; Guerinot, M.L. Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat. Chem. Biol. 2009, 5, 333–340.
- Wu, X.; Chen, J.; Yue, X.; Wei, X.; Zou, J.; Chen, Y.; Su, N.; Cui, J. The zinc-regulated protein (ZIP) family genes and glutathione s-transferase (GST) family genes play roles in Cd resistance and accumulation of pak choi (Brassica campestris ssp. chinensis). Ecotoxicol. Environ. Saf. 2019, 183, 109571.
- Wong, C.K.E.; Cobbett, C.S. HMA P-type ATPases are the major mechanism for root-to-shoot Cd translocation in Arabidopsis thaliana. New Phytol. 2009, 181, 71–78.
- Cun, P.; Sarrobert, C.; Richaud, P.; Chevalier, A.; Soreau, P.; Auroy, P.; Gravot, A.; Baltz, A.; Leonhardt, N.; Vavasseur, A. Modulation of Zn/Cd P (1B2)-ATPase activities in Arabidopsis impacts differently on Zn and Cd contents in shoots and seed. Metallomics 2014, 6, 2109–2116.
- Migocka, M.; Papierniak, A.; Maciaszczyk-Dziubinska, E.; Posyniak, E.; Kosieradzka, A. Molecular and biochemical properties of two P1B2-ATPases, CsHMA3 and CsHMA4, from cucumbe. Plant Cell Environ. 2014, 38, 1127–1141.
- Fleming, M.; Trenor, C.C.; Su, M.A.; Foernzler, D.; Beier, D.R.; Dietrich, W.F.; Andrews, N. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat. Genet. 1997, 16, 383–386.
- Chen, S.; Han, X.; Fang, J.; Lu, Z.; Qiu, W.; Liu, M.; Sang, J.; Jiang, J.; Zhuo, R. Sedum alfredii SaNramp6 Metal Transporter Contributes to Cadmium Accumulation in Transgenic Arabidopsis thaliana. Sci. Rep. 2017, 7, 13318.
- Wu, D.; Yamaji, N.; Yamane, M.; Kashino-Fujii, M.; Sato, K.; Ma, J.F. The HvNramp5 Transporter Mediates Uptake of Cadmium and Manganese, But Not Iron. Plant Physiol. 2016, 172, 1899–1910.
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 2019, 11, 255–277.
- Zhu, T.; Li, L.; Duan, Q.; Liu, X.; Chen, M. Progress in our understanding of plant responses to the stress of heavy metal cadmium. Plant Signal. Behav. 2021, 16, 1836884.
- Chen, C.; Li, Z.; Li, S.; Deng, N.; Mei, P. Effects of root exudates on the activation and remediation of cadmium ion in contaminated soils. Environ. SCI Pollut. Res. Int. 2020, 27, 2926–2934.
- Guo, J.; Xu, W.; Ma, M. The assembly of metals chelation by thiols and vacuolar compartmentalization conferred increased tolerance to and accumulation of cadmium and arsenic in transgenic Arabidopsis thaliana. J. Hazard. Mater. 2012, 199–200, 309–313.
- Anjum, N.A.; Hasanuzzaman, M.; Hossain, M.A.; Thangavel, P.; Roychoudhury, A.; Gill, S.S.; Rodrigo, M.A.M.; Adam, V.; Fujita, M.; Kizek, R.; et al. Jacks of metal/metalloid chelation trade in plants—An overview. Front. Plant Sci. 2015, 6, 192.
- Morris, C.A.; Nicolaus, B.; Sampson, V.; Harwood, J.L.; Kille, P. Identification and characterization of a recombinant metallothionein protein from a marine alga, Fucus vesiculosus. Biochem. J. 1999, 338, 553–560.
- Zhang, H.; Lv, S.; Xu, H.; Hou, D.; Li, Y.; Wang, F. H2O2 Is Involved in the Metallothionein-Mediated Rice Tolerance to Copper and Cadmium Toxicity. Int. J. Mol. Sci. 2017, 18, 10.
- Singh, G.; Tripathi, S.; Shanker, K.; Sharma, A. Cadmium-induced conformational changes in type 2 metallothionein of medicinal plant Coptis japonica: Insights from molecular dynamics studies of apo, partially and fully metalated forms. J. Biomol. Struct. Dyn. 2018, 37, 1520–1533.
- Vatamaniuk, O.K.; Mari, S.; Lu, Y.-P.; Rea, P.A. AtPCS1, a phytochelatin synthase from Arabidopsis: Isolation and in vitro reconstitution. Proc. Natl. Acad. Sci. USA 1999, 96, 7110–7115.
- Sharma, S.S.; Dietz, K.J.; Mimura, T. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ. 2016, 39, 1112–1126.
- Allan, D.L.; Jarrell, W.M. Proton and copper adsorption to maize and soybean root cell walls. Plant Physiol. 1989, 89, 823–832.
- He, J.; Qin, J.; Long, L.; Ma, Y.; Li, H.; Li, K.; Jiang, X.; Liu, T.; Polle, A.; Liang, Z.; et al. Net cadmium flux and accumulation reveal tissue-specific oxidative stress and detoxification in Populus × canescens. Physiol. Plant. 2011, 143, 50–63.
- Watanabe, M.; Suzuki, T. Involvement of reactive oxygen stress in cadmium-induced cellular damage in Euglena gracilis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 131, 491–500.
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.J.; et al. Cadmium stress: An oxidative challenge. Biometals 2010, 23, 927–940.
- Qiu, R.L.; Zhao, X.; Tang, Y.T.; Yu, F.M.; Hu, P.J. Antioxidative response to Cd in a newly discovered cadmium hyperaccumulator, Arabis paniculata F. Chemosphere 2008, 74, 6–12.
- Cai, S.-Y.; Zhang, Y.; Xu, Y.-P.; Qi, Z.-Y.; Li, M.-Q.; Ahammed, G.J.; Xia, X.-J.; Shi, K.; Zhou, Y.-H.; Reiter, R.J.; et al. HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plant. J. Pineal Res. 2017, 62, e12387.
- Chen, S.; Yu, M.; Li, H.; Wang, Y.; Lu, Z.; Zhang, Y.; Liu, M.; Qiao, G.; Wu, L.; Han, X.; et al. SaHsfA4c From Sedum alfredii Hance Enhances Cadmium Tolerance by Regulating ROS-Scavenger Activities and Heat Shock Proteins Expression. Front. Plant Sci. 2020, 11, 142.
- Zhao, C.; Peng, C.; Wang, P.; Fang, S.G.; Yan, L.L.; Qiu, L.H. Identification of co-chaperone Cdc37 in Penaeus monodon: Coordination with Hsp90 can reduce cadmium stress-induced lipid peroxidation. Ecotoxicol Environ. Safe 2021, 209, 111800.
- Shim, D.; Hwang, J.U.; Lee, J.; Lee, S.; Choi, Y.; An, G.; Martinoia, E.; Lee, Y. Orthologs of the class A4 heat shock transcription factor HsfA4a confer cadmium tolerance in wheat and rice. Plant Cell 2009, 21, 4031–4043.
- Kong, X.; Li, C.; Zhang, F.; Yu, Q.; Gao, S.; Zhang, M.; Tian, H.; Zhang, J.; Yuan, X.; Ding, Z. Ethylene promotes cadmium-induced root growth inhibition through EIN3 controlled XTH33 and LSU1 expression in Arabidopsis. Plant Cell Environ. 2018, 41, 2449–2462.
- Fuhrer, J. Ethylene Biosynthesis and Cadmium Toxicity in Leaf Tissue of Beans (Phaseolus vulgaris L.). Plant Physiol. 1982, 70, 162–167.
- Wang, Y.; Yuan, M.; Li, Z.; Niu, Y.; Jin, Q.; Zhu, B.; Xu, Y. Effects of ethylene biosynthesis and signaling on oxidative stress and antioxidant defense system in Nelumbo nucifera G. under cadmium exposure. Environ. Sci. Pollut. Res. 2020, 27, 40156–40170.
- Abozeid, A.; Ying, Z.; Lin, Y.; Liu, J.; Zhang, Z.; Tang, Z. Ethylene Improves Root System Development under Cadmium Stress by Modulating Superoxide Anion Concentration in Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 253.
- Jia, X.; Zhao, Y.H.; Liu, T.; He, Y.H. Leaf defense system of Robinia pseudoacacia L. seedlings exposed to 3years of elevated atmospheric CO (2) and Cd-contaminated soils. SCI Total Environ. 2017, 605–606, 48–57.
- Li, X.; Zhang, X.; Wu, Y.; Li, B.; Yang, Y. Physiological and biochemical analysis of mechanisms underlying cadmium tolerance and accumulation in turnip. Plant Divers. 2018, 40, 19–27.
- Alyemeni, M.N.; Ahanger, M.A.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Ahmad, P. Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 2018, 255, 459–469.
- Jiang, H.P.; Gao, B.B.; Li, W.H.; Zhu, M.; Zheng, C.F.; Zheng, Q.S.; Wang, C.H. Physiological and biochemical responses of Ulva prolifera and Ulva linza to cadmium stress. Sci. World J. 2013, 2013, 289537.
- Amri, B.; Khamassi, K.; Ali, M.B.; Jaime, A.; Teixeira, S.; Leila, B.B.K. Effects of gibberellic acid on the process of organic reserve mobilization in barley grains germinated in the presence of cadmium and molybdenum. S. Afr. J. Bot. 2016, 106, 35–40.
- Mihoub, A.; Chaoui, A.; El, F.E. Biochemical changes associated with cadmium and copper stress in germinating pea seeds (Pisum sativum L.). C R Biol. 2005, 328, 33–41.
