Polyelectrolyte multilayers are thin organic films obtained by self-assembly of PEs with other charged/uncharged (macro)molecules using an LbL method. The physical and chemical architecture of PEMs can be determined from the nanoscopic level to the macroscopic level. Both weak and strong PEs can participate in the formation of multilayers together with other low molecular or macromolecular compounds (charged or uncharged), the properties of the obtained materials being dependent on the nature and characteristics of the partners involved in the deposition process.
- layer-by-layer
- polyelectrolyte
- medical and environmental applications
- emerging pollutants
1. Introduction
Since the introduction of the layer-by-layer (LbL) deposition model by Iler in the 1960s [1] and its development by Decher and Hong in the 1990s [2], the deposition of polyelectrolyte multilayers was considered an effective method for modifying surfaces, regardless of their shape, and found many uses in top fields of science: controlled-release of bioactive compounds, tissue engineering, biosensors, environmental remediation, and pollutants retention, as well as the development of modern energy conversion and storage systems. Through their outstanding versatility, the surfaces functionalized with polyelectrolytes, especially with polyelectrolyte multilayers (PEMs), proved to offer suitable active colloidal supports for loading/release of bioactive compounds (drugs, proteins, enzymes, nucleic acids, aminoacids, etc.) or pollutants (dyes, pesticides, humic acids, heavy metal ions, etc.). Additionally, flat surfaces functionalized with polyelectrolyte multilayers can act as antimicrobial surfaces or, in the case of using natural polyelectrolytes, as supports for tissue engineering. Due to the high densities of functional groups bound to a flexible polymeric chain, polyelectrolytes have a great potential in biomedical and environmental applications through the intimate interaction at the atomic level with various types of active (bio)compounds. Taking the advantages of polyelectrolyte characteristics, numerous types of organic architectures can be fabricated by covalent or non-covalent bonding (hydrogen bonding, π-π stacking, and metal-ligand coordination, ionic and hydrophobic interactions) between polyelectrolytes and different inorganic/organic partners. This review aims to highlight the importance of polyelectrolyte multilayer assembly in solving two main problems of humans (increased number of sick people and less clean water to satisfy the economic and societal needs) by the creation of new formulations and advanced materials for drugs delivery and wastewater treatment, respectively [3][4][3,4]. The following sections present the general mechanism and methods used for LbL deposition of multilayers and the biomedical/environmental applications of PEMs.
2. Polyelectrolytes
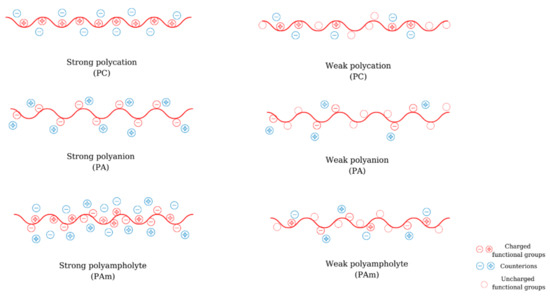
Polyelectrolytes (PEs) are macromolecular compounds that possess a minimum of 10–15% functional groups that are dissociated or dissociate in solution. Depending on their origin, polyelectrolytes can be natural (e.g., chitosan (CS), alginic acid, hyaluronic acid (HA), etc.), semi-synthetic (e.g., xanthan, modified CS), or synthetic (poly(ethyleneimine) (PEI), poly(acrylic acid) (PAA), poly(methacrylic acid) (PMA), etc. ) Depending on the nature of the ionic charge, polyelectrolytes can be classified into polyanions (PA) (polyacids, have negative charges), polycations (PC) (polybases, have positive charges), polyampholytes (PAm) (amphoteric, have both positive and negative charges) ( Figure 1 ). According to their dissociation behavior, PEs can be weak (their degree of ionization is depending on pH and their dissociation constant varies between 2 and 10) or strong (they dissociate completely in solution and their ionization degree is not affected by the pH).
To assess the degree of dissociation of a PE, potentiometric titrations can be performed, to obtain information about the acidity constant and the number of functional groups. Polyacids can be titrated with bases and polybases can be titrated with acids. The degree of dissociation of weak PEs is dependent on the number of functional groups in the macromolecular chain and can be calculated using the formula for the neutralization degree (Equation (1)): α = α ′ + C H + / C PE (1) where C H + is the molar concentration of H + ions and C PE is the molar mass of the PE.
The apparent acidity constant ( pK app ( PE ) ) can be calculated using a modified version of the Henderson-Hasselbalch Equation (2): pK app ( PE ) = pH - m · log ( α ′/( α ′−1)) (2) where m can be found from the experimentally performed titrations, as a slope of the curve pH = f ( log ( α ′/( α ′−1)) [5].
PEs can also be classified according to their composition (homopolymers and copolymers) or molecular architecture (linear, branched, or cross-linked). Ionizable functional groups of the PEs can be located on the main chain (“integral” PE) or on the side chain (“pendant” PE).
3. Fabrication Methods for Polyelectrolyte Multilayers
From the practical point of view, multilayers can be fabricated by several methods [6][7]: dipping, spray deposition, centrifugal deposition, electrodeposition, and microfluidic deposition, as summarized in Figure 23. The choice of the deposition method depends on the materials to be assembled, the time required for the deposition, and the shape of the substrate. PEs can be successfully deposited on various substrates, including flat and tridimensional materials, porous and microfluidic materials, and even biological molecules. Figure 23 presents the main methods used for the fabrication of PEMs on planar substrates (A–D) and microfluidic materials (E).
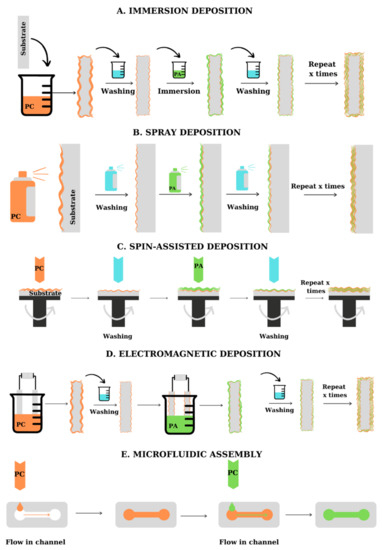
Spray-assisted deposition of polyelectrolytes is a quicker fabrication method and allows the fabrication of multilayers with a predetermined thickness, based on the change in deposition conditions, and can be applied to large substrates ( Figure 23 B) [7][16]. To facilitate the deposition of the PE layer, the spraying deposition is performed on supports placed perpendicular to the direction of application of the PE solution, which ensures the removal of excess deposited material due to gravitational drainage. The method was firstly proposed by Schlenoff et al. [8][17] which alternately sprayed poly(styrene sulfonate) (PSS) and poly(diallyldimethylammonium chloride) (PDADMAC) on support, obtaining LBL films with high uniformity and good quality. The thickness and roughness of the films obtained by spraying are lower than in the case of dipping deposition, but still, present an increasing mechanism dependent on the number of bilayers. By testing the deposition of PEs on a silica template using horizontal spray deposition, vertical spray deposition, and dipping deposition, Alongi et al. [9][18] observed that the best results in terms of film homogeneity were obtained using horizontal spray deposition. Dierendonck et al. [10][19] highlighted the recent developments in the spray-assisted deposition of PEs. As presented by the team, the PEMs obtained by spray deposition present a more regular surface. Hsu et al. [11][20] used the spray-assisted deposition of PEs for the encapsulation of lysozyme. Testing several variations for the assembly of polyelectrolytes, they optimized the deposition process, obtaining films with good enzyme encapsulation efficiency. The loss in raw material was also reduced. To avoid the deposition of an inhomogeneous film, the spraying method can be coupled with spinning deposition [12][13][8,14].
Microfluidic assembly of polyelectrolyte multilayers is used for the deposition of layers of polyelectrolytes on the walls and in the capillaries/channels of some microfluidic systems ( Figure 23 E). The alternating introduction of PEs solutions into the microfluidic assembly is usually carried out under vacuum or pressure. The method is faster than dipping deposition and allows the formation of polymeric films in materials with special forms for which other deposition methods cannot be applied [7][16]. From the beginning of the 2000s, several authors [14][15][16][28,29,30] reported the LbL assembly of PEs on microfluidic materials, highlighting the fast deposition and the uniformity of the multilayers obtained. Madaboosi et al. [16][30] used the microfluidic assembly method for the fabrication of poly-L-lysine (PLL)/HA films, observing that the PEs supply rate is one of the key parameters influencing the deposition. Wang et al. [17][31] proposed a model of electrodeposition and microfluidic coupling for LbL deposition of CS and ALG.
The methods presented above refer to the deposition of PEs on macroscopic surfaces. However, since many of the applications of PEMs are related to the deposition of PEs on substrates such as colloidal particles, liposomes, or biological molecules, it is necessary to develop methods for the deposition of charged compounds onto such templates. The general method involved in the fabrication of PEMs on microscopic or nanoscopic templates is the dipping deposition and its characteristics and control methods are similar to those presented above. The method is presented in more detail in Section 6.1.
4. Biomedical and Environmental Applications of Polyelectrolyte Multilayers
The incorporation of active compounds into multilayers ensures the improvement of stability and physico-chemical properties and protection of the active compound, allowing the release of the active substance in a controlled manner. As shown by Andreeva [18][71], the encapsulation of active compounds can ensure protection against different stress factors, such as exposure to light, humidity, or the human organism’s internal conditions by creating a barrier between the active compound and the environment. The possibility of modifying the release kinetics of active compounds by their encapsulation in multilayers is also important for controlled-release applications, based on the use of stimuli-responsive PEs as building blocks for the deposition of multilayers [19][125]. The swelling behavior of the PEMs can also influence the release of the active compound.
The incorporation of active compounds into multilayers can be achieved either by their retention inside multilayer capsules or by their adsorption within the multilayer, with the formation of (polyelectrolyte/active compound, AC) n systems. The multilayered systems for pharmaceuticals include porous nanoparticles, liposomes, thin films, microcapsules, and nanocapsules [20][126]. Table 1 presents some examples of active compounds incorporated into multilayers for facilitating their absorption.
| Active Compound | Therapeutic Class | Incorporation Method | Polyelectrolytes Used | Reference |
|---|---|---|---|---|
| Diclofenac, Indomethacin | Analgesic | PE-AC interaction | PEI–PAA | [21] |
| Ibuprofen | Analgesic | PE-AC interaction | CS–dextran sulphate CS–carboxymetylcelullose |
[22] |
| Rifampicin | Antibiotic | Incorporation into hollow capsules | PVP–PMA | [23] |
| Tetracycline | Antibiotic | PE-AC interaction | PAA–poly(L-lysine) | [24] |
| Gentamicin | Antibiotic | PE-AC interaction | PSS–PAH | [25] |
| Benzydamine | Anti-inflammatory agent | PE-AC interaction | CS–casein–poly(lactic acid) | [26] |
| Artemisin | Chemotherapeutic agent | PE-AC interaction | CS–gelatin–ALG | [27] |
| Paclitaxel Fluorouracil | Chemotherapeutic agent | PE-AC interaction | CS–dextran sulphate | [28] |
| Mitoxantrone | Chemotherapeutic agent | Incorporation into hollow capsules and PE-AC interaction | PLL–PEG–block-poly(L-aspartic acid)–liposomal nanoparticles | [29] |
| Doxorubicin | Chemotherapeutic agent | Incorporation into hollow capsules | CS–HA | [30] |
| Insulin | Hormone | PE-AC interaction | Poly(malic acid)–CS | [31] |
Bucatariu et al. [21][97] incorporated diclofenac sodium salt and indomethacin in a multilayer consisting of PEI and cross-linked with pyromellitic dianhydride and 3,3′, 4,4′-benzophenonetetracarboxylic dianhydride. The authors observed that the mass of active compounds retained in the multilayer was influenced by the number of layers of polycation deposited on the support and by the amount of cross-linker used. For the multilayer consisting of PEI and diclofenac, the amount of active substance retained was about 4 mg/g composite, while in the case of indomethacin, the amount of active adsorbed compound was about 5 mg/g composite.
