Highly pathogenic H5N1 and low pathogenic H9N2 avian influenza viruses are circulating in Bangladesh since 2007 causing serious economic losses to the country. Multiple virus introductions of different clades of HPAIV H5N1, reassorted genotypes, and on-going diversification of LPAIV H9N2 create a highly volatile virological environment which potentially implicates increased virulence, adaptation to new host species, and subsequent zoonotic transmission.
- Bangladesh, Poultry, Avian Influenza Virus
1. Definition
Infl
Influenza A viruses (IAVs), belonging to the family Orthomyxoviridae [1], are an important cause of respiratory infections of humans and many other species of mammals and birds. Avian influenza A viruses (AIV) are potentially zoonotic pathogens that infect a wide range of avian species and occasionally spill over into mammalian species, including humans [2][3][4]. IAVs contain a negative-sense segmented RNA genome. Their eight genome segments encode for at least ten classical influenza proteins: Polymerase basic 2 (PB2), Polymerase basic 1 (PB1), Polymerase acidic (PA), Hemagglutinin (HA), Nucleoprotein (NP), Neuraminidase (NA), Matrix 1 (M1), Matrix 2 (M2), Nonstructural 1 (NS1), and Nonstructural 2 or Nuclear Export Protein (NS2/NEP), as well as, dependent on the strain, a variable number of accessory proteins (e.g., PB1-F2, PB1-N40, PA-X, PA-N182, PA-N155) through frame-shifts and by use of complementary sequences [5][6][7]. AIVs are classified into subtypes based on their surface glycoproteins, HA and NA; there are currently 16 HA and 9 NA subtypes identified in avian species. Wild aquatic birds are the natural reservoirs of AIVs [8][9]. AIVs are further categorized by their pathogenicity in chickens into high and low pathogenicity avian influenza viruses (HPAIV, LPAIV). An intravenous pathogenicity index (IVPI) in chickens is used for biological pathotype characterization by experimental infection; alternatively, the sequence of an endoproteolytic cleavage site (CS) in the HA protein (HACS) can be used as a molecular marker of pathogenicity [10]. HPAI viruses exhibit high mortality in chickens and contain a polybasic HACS, which is recognized by endogenous and ubiquitous host cellular proteases, like furin, therefore predisposing these viruses to cause systemic, often lethal, infections. In contrast, LPAI viruses invoke mild respiratory illness but also run asymptomatic courses, especially in wild bird species. The LPAIV HACS consists of a mono-, di-, or tri-basic motif which restricts proteolytic cleavage activation to extracellular trypsin-like host proteases confined to the intestinal and respiratory epithelia, respectively [11]. LPAIVs are therefore incapable of inducing systemic infection.
2. Introduction
Aquatic birds arenza A the natural hosts of IAV. Sporadically, viruses (IAVs), belonging to the familycross from aquatic wild birds to poultry or mammals, and new, adapted viruses may become established in these spill-over hosts. AIVs of subtypes OrthomyxoviridaeH5 and [1],H7 are anthe most important cause of respiratory infectiones that cross to terrestrial poultry. These viruses have proven ability to mutate in poultry from low pathogenicity (LP) precursors circulating in wild birds into high pathogenicity (HP) viruses that multiply systemically in chickens, often causing very high mortality in infected flocks [12]. The factonrs of humans and many other species of mammals and birdsgoverning such molecular mutation events are not fully understood. Therefore, infections of poultry with AIV of subtypes H5 and H7 are notifiable and require obligatory restriction measures [12][13]. The zoonotic Avian influenza A/goose/Guangdong/1/96 (gs/GD) lineage of H5N1 HPAI viruses (AIV) are potentially zoonotic, along with the G1 lineage of H9N2 and the Chinese H7N9 AIV, became well adapted to poultry and endemically circulate in many countries and in China, respectively [13][14][15][16]. Multiple clathogens that infecdes/lineages and sub-lineages within these subtypes have been recognized, indicating ongoing evolution with significant genetic drift a [17][18][19].
Worldwide, range of avian species and occasionallBangladesh is among the countries with the highest number of reported HPAI outbreaks in poultry [20]. Thisp ill over into mammalian s due to repeated incursions and endemic spread in poultry of HPAIV H5N1 of the gs/GD lineage since 2007 [21]. Sporadically, thespe HPAIVs are also detecies, including humansted in wild birds in Bangladesh [2,3,4][22][23]. LPAIAVs contain a negativV H9N2 was first detected in the country in 2006 and has likewise become endemic in poultry and is co-circulating in the country, together with HPAIV [24][25]. Furthe-r AIV sense segmented RNA genomeubtypes were isolated intermittently from domestic free-range birds and, more rarely, from aquatic wild birds [22][23][26]. The wir eight genome segments encode for at least ten classical influenza protdespread continuous co-circulation of HPAIV H5N1 and LPAIV H9N2 bears increased risks for the potential generation of new sub- and genotypes of AIVs which constitute additional obstacles to virus eradication. Both viruses cause significant economic damage in poultry production and threaten public health by their zoonotic propensity [27]. AIV surveillans: Polymerce studies in Bangladesh [22][26][28][29] have se basic 2 (PB2), Polymerase bashown that domestic ducks play an important role in the transmission and emergence of new AIV sub- and genotypes.
3. Ecology and Epidemiology of AIVs in Bangladesh
3.1. Geographical and Ecological Frameworks
Bangladesh ics 1 (PB1), Polymerase acidic (PA), Hemagglutinin (HA), Nucleoprotein (NP), Neuraminidase (NA), Matrix 1 (M1), Matrix 2 (M2), Nonstructural 1 (NS1), and Nonstructural 2 or Nuclear Export Protein (NS2/NEP), as well as, depa low to middle-income country in South Asia agriculturally characterized by rich water environments, paddy rice farming, and poultry production. The economy is heavily dependent on agriculture and livestock production. The country consists of a broad, deltaic plain with many tributaries and a sea coast with an extensive mangrove belt. It is at high risk of frequent flooding by three major rivers, the Ganges, Jamuna, and Brahmaputra. In addition, there is annual flooding from the seaside due to cyclones in the Bengal Bay of the Indian Ocean. Bangladesh is also an attractive and important wintering site for wild migratory birds, in particular, of the order Anseriformes, which breed indent on the strain, a variable arctic and palearctic regions of Russia. Moreover, two major migratory bird flyways, the Central Asian and East Asian-Australian, are crossing Bangladesh [30][31][32]. The abunumbdancer of accessory proteins (e.g., PB1-F2, PB1-N40, PA-X, PA-N182, PA-N155) through frame-s of shallow coastal wetlands and vast inland wetlands (so-called haors) provide a large reservoir for wildlife, especially waterfowl, which migrate from many parts of Russia and Central Asia during winter [22][33]. Thifs creats and by use of complementary sequences [5,6,7].es an ecological scenario where wild aquatic birds, domestic ducks and galliform poultry intermingle and in which pathogens, like AIVs, are classified into subtypes based on their surface glycoproteins, HA and NA; there are currcan be easily exchanged (Figure 1). According to farmers’ complains, the outbreaks are more common during the autumn and winter; however, studies were unable to identify any distinct seasonality for endemic H5N1 and H9N2 virus outbreaks in Bangladesh, and AIVs have been frequently 16 HA and 9 NA subtypeidentified from poultry in live bird markets (LBM) throughout the year [34][35][36][37]. This indentified in aviicates that endemic virus circulation in poultry populations is likely the most important driver in this scenario.
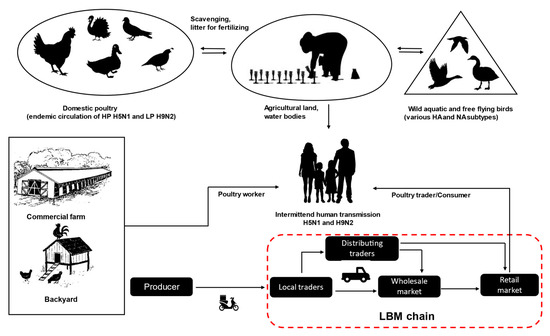
Figure 1. An overview of avian influenza virus transmission and live bird market trading chains (red dashed line) in Bangladesh.
3.2. Poultry Rearing Systems and Trading Chains
Poultry rearin species. Wild aquatic birds are the natug in Bangladesh comprises commercial and backyard poultry production. Commercial farming can be further categorized according to farm sizes following the sectoring approach of the Food and Agriculture Organization of the United States (FAO) [38]: large-scal reservoirs of AIVs [8e breeder farming, medium size farming (broiler, layer, and duck), and small size farming (layer, broiler, quail, pigeon, turkey, guinea fowl,9] etc.). AIVs are furn Bangladesh, more than 60% of the people live in rural villages [39], and it is estimated ther categorized by tat about 90% of the rural households raise poultry (chicken, duck, pigeon) in traditional backyard settings [40]. The mair pathogenicity inn poultry types reared and traded in the country include: Industrial white-feathered broiler chickens [38], Sonalinto h (cross-breed of Rhode Island Red cocks and Fayoumi hens) [41], Deshig (back-yard chickens) [42], and ducks. Broilow pathogeer, Sonali, and few ducks are raised in commercial settings, while Deshi and most of the ducks are reared in traditional scavenging systems [43][44]. Many further commercicity avian influenza virusal poultry breeds, such as hybrid layer, have successfully been established in recent years in Bangladesh and are being profitably utilized by different entrepreneurs [45].
At the beginning of 21s (HPAIV, LPAIV). An intravenous pathogenicity index (IVPI) in chickens is used for biological pathotype characterization by ext century, the Bangladeshi poultry industry expanded rapidly. The Government of Bangladesh has given top priority to livestock development to meet the growing demand for high quality animal protein in the human diet and to create employment opportunities and generate income for the low-income rural population. As such, both industrial poultry production and family poultry rearing are supported. Bangladesh currently raises an estimated 282 million of chickens and 55 million of ducks [46]. Improperi managemental infection; alternatively, the seq and biosecurity practices in poultry rearing have fostered the emergence and re-emergence of economically important infectious disease, like HPAI, leading to endemic spread of HPAIV H5N1 and LPAIV H9N2 [27][47]. During thence of an endoproteolytic cle first and second wave of HPAI H5N1 in 2007 and 2008, approximately 547 poultry farms had been affected that forced the authority to cull nearly 1.7 million birds, resulting in substantial financial losses [48]. Agavinst the general market rules where shortage site (CS) in the HA protein (HACS) can be used as a molecof products induces rising prices, the price of poultry meat and eggs in Bangladesh declined by 27% as a majority of the consumers desisted from consumption of potentially unsafe broiler meat and chicken eggs [48]. Finally, the poultry mar marker of patket collapsed, and many farm owners lost all capital.
LBMs hogave beenicity [10]. incriminated in the dynamics of HPAI viruses exhibit high mortality in chickens and contaVs transmission, dissemination, and persistent circulation, thus facilitating the reassortment between different virus strains in many countries [49][50]. In LBMs, mixing of a polybasic HACS, which is recognized by endogenous and different species of birds (chickens, ducks, geese, pigeons, etc.) from different sources (wild birds, backyards, and commercial farms) creates a suitable niche for persistence and perpetuation of AIVs. In Bangladesh, live bird trading is ubiquitous host cellular, and LBMs distribute 95% of the total poultry meat and egg retails p[38]. Birds are moteases, like furin, therefore predisposstly traded alive because of cultural and religious preferences for consuming freshly slaughtered poultry. There is an apparent lack of processed meat marketing facilities and cold chains, particularly among rural households [51]. LBMs in rural Bang these viruses to cause systemic, often lethal, infections. In contrast, LPAI virladesh often do not provide even a minimum level of biosecurity and lack proper disinfection and sanitation procedures. Before finally sold in LBMs, birds often move through a complex trading network of peripheral rural markets via several transshipment stations to wholesalers in city markets, thereby increasing risks for AIV spread and transmission to humans (Figure 1). Since 2008, several subtypes of AIVs, including, predominantly, HPAIV H5N1 and LPAIV H9N2, have been isolated from LBMs in Bangladesh [34][35][36][50]. Unhygienic slaughtering processes inat LBMs bear increased risks of zoonotic transmissions [34][50], but actual virus transmissioke mild resn to LBM workers as evidenced by seroconversion was scarce [52]. Yet, LBMs remain a very impioratory illness but also run asymptomatic coursestant target to understand and intercept the local circulation of AIVs in domestic poultry in Bangladesh and to identify and combat risk factors in zoonotic transmission. The outbreak frequency of endemic HPAI H5N1 and LPAI H9N2 has increased due to increased poultry production, characterized by a mélange of various, highly fragmented rearing systems and marketing chains [53], easpecia elucidated above.
3.3. Virus Transmission and Risk Factors
Droplet, aerosoly in wild bird species. The LPAIV, faeco-oral, and direct or indirect contact with contaminated materials are widely described modes of transmission of AIVs HACS[54]. cTransmissionsists of a mono- risks increase depending on host susceptibility and viral load in the environment, as well as on distance between and frequency of contacts [55]. In Bangladesh, the di-, or tri-basic motif which romestic duck is currently considered as the most important epidemiological factor associated with AIV transmission between wild birds and other poultry [22][56]. Low awarenesstricts proteolytic cleavage activation to among the raisers of backyard poultry of the zoonotic properties of AIV, neglected practice of biosecurity measures, and the close living arrangements of poultry and rural human populations lodge them at the highest risk for zoonotic transmission [27]. HPAI H5N1 and exLPAI H9N2 outracellular trypsbreaks cause enormous losses to small scale poultry producers [57]. Safe disposal of litter an-like host proteases confined to the intd fallen animals pose further severe problems. Untreated poultry litters are being used as fertilizer on agricultural lands and as fish feed in water bodies, which may contaminate the environment and further trigger viral spreading [58]. However, large-scale commercial farmstinal and res are relatively better managed and follow biosecurity recommendations, which reduces huge financial losses due to AI outbreaks [27]. Moreover, LBMs proviratory epithelia, rde foraging opportunities for peri-domestic birds, such as crows, sparrows, and starlings [59]. Consequently, houspectively [11].e crows have been found positive for LHPAIVs a H5N1 on several occasions [60]. Moreover, therefore incapable of inducno closure days for LBMs with H5 AIV-positive birds are usually decreed. Possible risk factors stratified according to poultry rearing and marketing systemic infections in Bangladesh are listed in Table 1. Effective human-human transmission of HPAI or LPAI virus is not evident in the country, but avian–human transmission follows the close proximity of each other [27][50].
2. Introduction
3. Ecology and Epidemiology of AIVs in Bangladesh
3.1. Geographical and Ecological Frameworks

3.2. Poultry Rearing Systems and Trading Chains
3.3. Virus Transmission and Risk Factors
Risk factors identified in the Bangladeshi poultry farming and marketing systems that may promote spread of avian influenza virus (AIV).
| Sectors | Possible Risk Factors | References |
|---|
| Backyard poultry |
| [27, | [27] | 40, | [40] | 43, | [43] | 61, | [61] | 62, | [62] | 63, | [63] | 64, | [ | 65,66, | 64 | 67] | ][65][66][67] | ||
| Commercial poultry | |||||||||||||||||||||
| Small and medium enterprises |
| [21,39,43,57,68,69] | [21][39][43][57][68][69] | ||||||||||||||||||
| Large holding (specifically, commercial layer farms) |
| [21,70] | [21][70] | ||||||||||||||||||
| Live bird markets (LBM) |
| [21,27,39, | ] | 57,59, | [ | 61,71, | 57][59][61] | 72] | [21][27][39[71][72] | ||||||||||||
References
- Knipe, D.M.; Howley, P.M.; Fields, B.N. Fields Virology, 5th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; Chapter 47; pp. 1647–1689. [Google Scholar]
- Alexander, D.J. Summary of avian influenza activity in Europe, Asia, Africa, and Australasia, 2002–2006. Avian Dis. 2007, 51, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.S.; Yuen, K.Y. Avian influenza virus infections in humans. Chest 2006, 129, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Herfst, S.; Imai, M.; Kawaoka, Y.; Fouchier, R.A. Avian influenza virus transmission to mammals. Curr. Top. Microbiol. Immunol. 2014, 385, 137–155. [Google Scholar] [CrossRef]
- Ghedin, E.; Sengamalay, N.A.; Shumway, M.; Zaborsky, J.; Feldblyum, T.; Subbu, V.; Spiro, D.J.; Sitz, J.; Koo, H.; Bolotov, P.; et al. Large-scale sequencing of human influenza reveals the dynamic nature of viral genome evolution. Nature 2005, 437, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Revol, R.; Östbye, H.; Wang, H.; Daniels, R. Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef] [PubMed]
- Jagger, B.W.; Wise, H.M.; Kash, J.C.; Walters, K.A.; Wills, N.M.; Xiao, Y.L.; Dunfee, R.L.; Schwartzman, L.M.; Ozinsky, A.; Bell, G.L.; et al. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science 2012, 337, 199–204. [Google Scholar] [CrossRef]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef]
- Fouchier, R.A.; Munster, V.; Wallensten, A.; Bestebroer, T.M.; Herfst, S.; Smith, D.; Rimmelzwaan, G.F.; Olsen, B.; Osterhaus, A.D. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 2005, 79, 2814–2822. [Google Scholar] [CrossRef]
- OIE. Influenza A Cleavage Site; World Organization for Animal Health (OIE): Paris, France, 2014; Available online: https://www.oie.int/doc/ged/D13484.PDF (accessed on 2 May 2020).
- Böttcher-Friebertshäuser, E.; Garten, W.; Matrosovich, M.; Klenk, H.D. The hemagglutinin: A determinant of pathogenicity. Curr. Top. Microbiol. 2014, 385, 3–34. [Google Scholar] [CrossRef]
- Alexander, D.J. A review of avian influenza in different bird species. Vet. Microbiol. 2000, 74, 3–13. [Google Scholar] [CrossRef]
- Guo, Y.J.; Krauss, S.; Senne, D.A.; Mo, I.P.; Lo, K.S.; Xiong, X.P.; Norwood, M.; Shortridge, K.F.; Webster, R.G.; Guan, Y. Characterization of the pathogenicity of members of the newly established H9N2 influenza virus lineages in Asia. Virology 2000, 267, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Shortridge, K.F.; Krauss, S.; Webster, R.G. Molecular characterization of H9N2 influenza viruses: Were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc. Natl. Acad. Sci. USA 1999, 96, 9363–9367. [Google Scholar] [CrossRef]
- Suarez, D.L.; Perdue, M.L.; Cox, N.; Rowe, T.; Bender, C.; Huang, J.; Swayne, D.E. Comparisons of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Kong. J. Virol. 1998, 72, 6678–6688. [Google Scholar] [CrossRef]
- Lam, T.T.; Wang, J.; Shen, Y.; Zhou, B.; Duan, L.; Cheung, C.L.; Ma, C.; Lycett, S.J.; Leung, C.Y.; Chen, X.; et al. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature 2013, 502, 241–244. [Google Scholar] [CrossRef]
- Smith, G.J.; Donis, R.O. Nomenclature updates resulting from the evolution of avian influenza A(H5) virus clades 2.1.3.2a, 2.2.1, and 2.3.4 during 2013–2014. Influenza Other Respir. Viruses 2015, 9, 271–276. [Google Scholar] [CrossRef]
- Pusch, E.A.; Suarez, D.L. The Multifaceted Zoonotic Risk of H9N2 Avian Influenza. Vet. Sci. 2018, 5, 82. [Google Scholar] [CrossRef]
- Zhuang, Q.; Wang, S.; Liu, S.; Hou, G.; Li, J.; Jiang, W.; Wang, K.; Peng, C.; Liu, D.; Guo, A.; et al. Diversity and distribution of type A influenza viruses: An updated panorama analysis based on protein sequences. Virol. J. 2019, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.S.; Ersbøll, A.K.; Biswas, P.K.; Christensen, J.P.; Hannan, A.S.; Toft, N. Ecological determinants of highly pathogenic avian influenza (H5N1) outbreaks in Bangladesh. PLoS ONE 2012, 7, e33938. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.K.; Christensen, J.P.; Ahmed, S.S.; Barua, H.; Das, A.; Rahman, M.H.; Giasuddin, M.; Hannan, A.S.; Habib, M.A.; Ahad, A.; et al. Avian influenza outbreaks in chickens, Bangladesh. Emerg. Infect. Dis. 2008, 14, 1909–1912. [Google Scholar] [CrossRef]
- Barman, S.; Marinova-Petkova, A.; Hasan, M.K.; Akhtar, S.; El-Shesheny, R.; Turner, J.C.; Franks, J.; Walker, D.; Seiler, J.; Friedman, K.; et al. Role of domestic ducks in the emergence of a new genotype of highly pathogenic H5N1 avian influenza A viruses in Bangladesh. Emerg. Microbes Infect. 2017, 6, e72. [Google Scholar] [CrossRef]
- Yang, G.; Chowdury, S.; Hodges, E.; Rahman, M.Z.; Jang, Y.; Hossain, M.E.; Jones, J.; Stark, T.J.; Di, H.; Cook, P.W.; et al. Detection of highly pathogenic avian influenza A(H5N6) viruses in waterfowl in Bangladesh. Virology 2019, 534, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Jannat, N.; Chowdhury, E.H.; Parvin, R.; Begum, J.A.; Khan, M.A.H.N.A.; Islam, M.R. Investigation of an outbreak of low pathogenic avian influenza in poultry in Bangladesh. Int. J. Livest. Res. 2013, 3, 21–32. [Google Scholar]
- Parvin, R.; Heenemann, K.; Halami, M.Y.; Chowdhury, E.H.; Islam, M.R.; Vahlenkamp, T.W. Full-genome analysis of avian influenza virus H9N2 from Bangladesh reveals internal gene reassortments with two distinct highly pathogenic avian influenza viruses. Arch. Virol. 2014, 159, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Sarker, R.D.; Giasuddin, M.; Chowdhury, E.H.; Islam, M.R. Serological and virological surveillance of avian influenza virus in domestic ducks of the north-east region of Bangladesh. BMC Vet. Res. 2017, 13, 180. [Google Scholar] [CrossRef]
- Rimi, N.A.; Hassan, M.Z.; Chowdhury, S.; Rahman, M.; Sultana, R.; Biswas, P.K.; Debnath, N.C.; Islam, S.S.; Ross, A.G. A Decade of Avian Influenza in Bangladesh: Where Are We Now? Trop. Med. Infect. Dis. 2019, 4, 119. [Google Scholar] [CrossRef]
- Khatun, A.; Giasuddin, M.; Islam, K.M.; Khanom, S.; Samad, M.A.; Islam, M.R.; Noor, M.; Bhuiyan, J.U.; Kim, W.I.; Eo, S.K.; et al. Surveillance of avian influenza virus type A in semi-scavenging ducks in Bangladesh. BMC Vet. Res. 2013, 9, 196. [Google Scholar] [CrossRef]
- Parvin, R.; Kabiraj, C.K.; Mumu, T.T.; Chowdhury, E.H.; Islam, M.R.; Beer, M.; Harder, T. Active virological surveillance in backyard ducks in Bangladesh: Detection of avian influenza and gammacoronaviruses. Avian Pathol. 2020, 1–8. [Google Scholar] [CrossRef]
- Parvin, R.; Kamal, A.H.; Haque, M.E.; Chowdhury, E.H.; Giasuddin, M.; Islam, M.R.; Vahlenkamp, T.W. Genetic characterization of highly pathogenic H5N1 avian influenza virus from live migratory birds in Bangladesh. Virus Genes 2014, 49, 438–448. [Google Scholar] [CrossRef]
- Takekawa, J.Y.; Prosser, D.J.; Collins, B.M.; Douglas, D.C.; Perry, W.M.; Yan, B.; Ze, L.; Hou, Y.; Lei, F.; Li, T.; et al. Movements of wild ruddy shelducks in the Central Asian Flyway and their spatial relationship to outbreaks of highly pathogenic avian influenza H5N1. Viruses 2013, 5, 2129–2152. [Google Scholar] [CrossRef]
- Palm, E.C.; Newman, S.H.; Prosser, D.J.; Xiao, X.; Ze, L.; Batbayar, N.; Balachandran, S.; Takekawa, J.Y. Mapping migratory flyways in Asia using dynamic Brownian bridge movement models. Mov. Ecol. 2015, 3, 3. [Google Scholar] [CrossRef]
- Lepage, D. Avibase. 2015. Available online: http://avibase.bsc-eoc.org/checklist.jsp?lang=EN®ion=bd&list=clements (accessed on 12 May 2020).
- Marinova-Petkova, A.; Shanmuganatham, K.; Feeroz, M.M.; Jones-Engel, L.; Hasan, M.K.; Akhtar, S.; Turner, J.; Walker, D.; Seiler, P.; Franks, J.; et al. The Continuing Evolution of H5N1 and H9N2 Influenza Viruses in Bangladesh Between 2013 and 2014. Avian Dis. 2016, 60, 108–117. [Google Scholar] [CrossRef]
- Shanmuganatham, K.; Feeroz, M.M.; Jones-Engel, L.; Smith, G.J.; Fourment, M.; Walker, D.; McClenaghan, L.; Alam, S.M.; Hasan, M.K.; Seiler, P.; et al. Antigenic and molecular characterization of avian influenza A(H9N2) viruses, Bangladesh. Emerg. Infect. Dis. 2013, 19, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Gerloff, N.A.; Khan, S.U.; Zanders, N.; Balish, A.; Haider, N.; Islam, A.; Chowdhury, S.; Rahman, M.Z.; Haque, A.; Hosseini, P.; et al. Genetically Diverse Low Pathogenicity Avian Influenza A Virus Subtypes Co-Circulate among Poultry in Bangladesh. PLoS ONE 2016, 11, e0152131. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Gurley, E.S.; Gerloff, N. Avian influenza surveillance in domestic waterfowl and environment of live bird markets in Bangladesh, 2007–2012. Sci. Rep. 2018, 8, 9396. [Google Scholar] [CrossRef] [PubMed]
- Dolberg, F. Poultry Sector Country Overview: Bangladesh. FAO Animal Production and Health Division, 2008. Available online: http://www.fao.org/3/a-ai319e.pdf (accessed on 2 May 2020).
- The World Bank. Agriculture & Rural Development. 2013. Available online: http://data.worldbank.org/topic/agriculture-and-rural-development?display=graph (accessed on 12 April 2020).
- Sultana, R.; Nahar, N.; Rimi, N.A.; Azad, S.; Islam, M.S.; Gurley, E.S.; Luby, S.P. Backyard poultry raising in Bangladesh: A valued resource for the villagers and a setting for zoonotic transmission of avian influenza. A qualitative study. Rural Remote Health 2012, 12, 1927. [Google Scholar] [PubMed]
- FAO. Comparative Performance of Sonali Chickens, Commercial Broilers, Layersand Local Non-descript (deshi) Chickens in Selected Areas of Bangladesh; Food and Agriculture Organization (FAO): Rome, Italy, 2015; Available online: www.fao.org/3/a-i4725e.pdf (accessed on 10 April 2020).
- Bhuiyan, A.K.F.H.; Bhuiyan, M.S.A.; Deb, G.K. Indigenous chicken genetic resources in Bangladesh: Current status and future outlook. Anim. Genet. Resour. Inf. 2005, 36, 73–84. [Google Scholar] [CrossRef]
- Biswas, P.K.; Christensen, J.P.; Ahmed, S.S.; Barua, H.; Das, A.; Rahman, M.H.; Giasuddin, M.; Hannan, A.S.; Habib, A.M.; Debnath, N.C. Risk factors for infection with highly pathogenic influenza A virus (H5N1) in commercial chickens in Bangladesh. Vet. Rec. 2009, 164, 743–746. [Google Scholar] [CrossRef]
- Biswas, P.K.; Christensen, J.P.; Ahmed, S.S.; Das, A.; Rahman, M.H.; Barua, H.; Giasuddin, M.; Hannan, A.S.; Habib, M.A.; Debnath, N.C. Risk for infection with highly pathogenic avian influenza virus (H5N1) in backyard chickens, Bangladesh. Emerg. Infect. Dis. 2009, 15, 1931–1936. [Google Scholar] [CrossRef]
- Huque, K.; Khan, M. Socio-geographic distribution of livestock and poultry in Bangladesh—A review. Bangladesh J. Anim. Sci. 2017, 46, 65–81. [Google Scholar] [CrossRef]
- DLS. Livestock Economy at a Glance, Government of Bangladesh. Department of Livestock Services (DLS), Bangladesh. 2018. Available online: http://dls.portal.gov.bd/sites/default/files/files/dls.portal.gov.bd/page/ee5f4621_fa3a_40ac_8bd9_898fb8ee4700/Livestock%20Economy%20at%20a%20glance%20%20%282017–2018%29.pdf (accessed on 24 April 2020).
- Parvin, R.; Begum, J.A.; Nooruzzaman, M.; Chowdhury, E.H.; Islam, M.R.; Vahlenkamp, T.W. Review analysis and impact of co-circulating H5N1 and H9N2 avian influenza viruses in Bangladesh. Epidemiol. Infect. 2018, 146, 1259–1266. [Google Scholar] [CrossRef]
- Alam, J.; Giasuddin, M.; Samad, M.A.; Taimur, M.J.F.A. Recent evidence of Avian Influenza in Bangladesh: A review. World Poult. Sci. J. 2010, 66, 455–464. [Google Scholar] [CrossRef]
- Kim, S.H. Challenge for One Health: Co-Circulation of Zoonotic H5N1 and H9N2 Avian Influenza Viruses in Egypt. Viruses 2018, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.C.; Feeroz, M.M.; Hasan, M.K.; Akhtar, S.; Walker, D.; Seiler, P.; Barman, S.; Franks, J.; Jones-Engel, L.; McKenzie, P.; et al. Insight into live bird markets of Bangladesh: An overview of the dynamics of transmission of H5N1 and H9N2 avian influenza viruses. Emerg. Microbes Infect. 2017, 6, e12. [Google Scholar] [CrossRef] [PubMed]
- Moyen, N.; Ahmed, G.; Gupta, S.; Tenzin, T.; Khan, R.; Khan, T.; Debnath, N.; Yamage, M.; Pfeiffer, D.U.; Fournie, G. A large-scale study of a poultry trading network in Bangladesh: Implications for control and surveillance of avian influenza viruses. BMC Vet. Res. 2018, 14, 12. [Google Scholar] [CrossRef]
- Nasreen, S.; Khan, S.U.; Luby, S.P.; Gurley, E.S.; Abedin, J.; Zaman, R.U.; Sohel, B.M.; Rahman, M.; Hancock, K.; Levine, M.Z.; et al. Highly pathogenic Avian Influenza A(H5N1) virus infection among workers at live bird markets, Bangladesh, 2009–2010. Emerg. Infect. Dis. 2015, 21, 629–637. [Google Scholar] [CrossRef]
- FAO. Approaches to Controlling, Preventing and Eliminating H5N1 Highly Pathogenic Avian Influenza in Endemic Countries. 2011. Available online: http://www.fao.org/3/i2150e/i2150e00.htm (accessed on 12 March 2020).
- Killingley, B.; Nguyen-Van-Tam, J. Routes of influenza transmission. Influenza Other Respir. Viruses 2013, 7, 42–51. [Google Scholar] [CrossRef]
- Peacock, T.H.P.; James, J.; Sealy, J.E.; Iqbal, M. A Global Perspective on H9N2 Avian Influenza Virus. Viruses 2019, 11, 620. [Google Scholar] [CrossRef]
- Parvin, R.; Begum, J.A.; Chowdhury, E.H.; Islam, M.R. Co-subsistence of avian influenza virus subtypes of low and high pathogenicity in Bangladesh: Challenges for diagnosis, risk assessment and control. Sci. Rep. 2019, 9, 8306. [Google Scholar] [CrossRef]
- Rimi, N.A.; Sultana, R.; Muhsina, M.; Uddin, B.; Haider, N.; Nahar, N.; Zeidner, N.; Sturm-Ramirez, K.; Luby, S.P. Biosecurity Conditions in Small Commercial Chicken Farms, Bangladesh 2011–2012. Ecohealth 2017, 14, 244–258. [Google Scholar] [CrossRef]
- Sarker, B.C.; Alam, M.A.; Rahman, M.M.; Islam, A.F.M.T.; Chowdhury, M.G.F. Waste management of commercial poultry farms in Bangladesh. J. Innov. Dev. Strateg. 2009, 3, 34–37. [Google Scholar]
- Biswas, P.K.; Giasuddin, M.; Nath, B.K.; Islam, M.Z.; Debnath, N.C.; Yamage, M. Biosecurity and Circulation of Influenza A (H5N1) Virus in Live-Bird Markets in Bangladesh, 2012. Transbound. Emerg. Dis. 2017, 64, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Berman, L.; Haider, N.; Gerloff, N.; Rahman, M.Z.; Shu, B.; Rahman, M.; Dey, T.K.; Davis, T.C.; Das, B.C.; et al. Investigating a crow die-off in January-February 2011 during the introduction of a new clade of highly pathogenic avian influenza virus H5N1 into Bangladesh. Arch. Virol. 2014, 159, 509–518. [Google Scholar] [CrossRef] [PubMed]
- SAPPLPP. Combating Bird Flu through Bio-security Measures at Farm and Community Level: Evidence from Bangladesh; Good Practice Note: Delhi, India, 2010; Available online: http://sapplpp.org/publications/good-practice-notes-briefs/small-holder-poultry/BDGP03-combating-bird-flu-through-bio-security-measures.html#.XvRGsigzbIU (accessed on 24 March 2020).
- Sultana, R.; Rimi, N.A.; Azad, S.; Islam, M.S.; Khan, M.S.; Gurley, E.S.; Nahar, N.; Luby, S.P. Bangladeshi backyard poultry raisers’ perceptions and practices related to zoonotic transmission of avian influenza. J. Infect. Dev. Countr. 2012, 6, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Rimi, N.A.; Sultana, R.; Ishtiak-Ahmed, K.; Rahman, M.Z.; Hasin, M.; Islam, M.S.; Azziz-Baumgartner, E.; Nahar, N.; Gurley, E.S.; Luby, S.P. Understanding the failure of a behavior change intervention to reduce risk behaviors for avian influenza transmission among backyard poultry raisers in rural Bangladesh: A focused ethnography. BMC Public Health 2016, 16, 858. [Google Scholar] [CrossRef] [PubMed]
- Popy, F.Y.; Chowdhury, Q.; Alam, S.; Roy, S.; Dipta, P.M.; Ahmed, J. Backyard Poultry Management and Production System at Barlekha Upazila, Moulvibazar, Bangladesh. Int. J. Sci. Bus. 2018, 2, 90–100. [Google Scholar]
- Conan, A.; Goutard, F.L.; Sorn, S.; Vong, S. Biosecurity measures for backyard poultry in developing countries: A systematic review. BMC Vet. Res. 2012, 8, 240. [Google Scholar] [CrossRef]
- Shanta, I.S.; Hasnat, M.A.; Zeidner, N.; Gurley, E.S.; Azziz-Baumgartner, E.; Sharker, M.A.Y.; Hossain, K.; Khan, S.U.; Haider, N. Raising Backyard Poultry in Rural Bangladesh: Financial and Nutritional Benefits, but Persistent Risky Practices. Transbound. Emerg. Dis. 2017, 64, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Rimi, N.A.; Sultana, R.; Ishtiak-Ahmed, K.; Haider, N.; Azziz-Baumgartner, E.; Nahar, N.; Luby, S.P. Where backyard poultry raisers seek care for sick poultry: Implications for avian influenza prevention in Bangladesh. BMC Public Health 2018, 18, 969. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Akhter, M.; Mamun, S.; Chowdhury, E.H.; Das, P.M. Bio-security in small scale poultry farms against avian influenza: Knowledge, attitude and practices. Asian J. Med. Biol. Res. 2016, 1, 670–676. [Google Scholar] [CrossRef]
- Islam, M.; Huque, Q. Practices of bio-security in small-scale broiler farms. Bangladesh Vet. 2007, 24, 72–78. [Google Scholar]
- Osmani, M.G.; Ward, M.P.; Giasuddin, M.; Islam, M.R.; Kalam, A. The spread of highly pathogenic avian influenza (subtype H5N1) clades in Bangladesh, 2010 and 2011. Prev. Vet. Med. 2014, 114, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.P.; Tardif-Douglin, D.; Ryan-Silva, R.; Magnani, R. Controlling highly pathogenic avian influenza, Bangladesh. Emerg. Infect. Dis. 2012, 18, 2083–2085. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, M.A.; Smallwood, C.; Imam, T.; Mahmud, R.; Hasan, R.B.; Hasan, M.; Anwer, M.S.; Rashid, M.H.; Hoque, M.A. Assessment of hygienic conditions of live bird markets on avian influenza in Chittagong metro, Bangladesh. Prev. Vet. Med. 2017, 142, 7–15. [Google Scholar] [CrossRef] [PubMed]
References
- Knipe, D.M.; Howley, P.M., Fields, B.N. Fields Virology, 5th Edition; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; Chapter 47, pp.1647–1689.
- Alexander, D.J. Summary of avian influenza activity in Europe, Asia, Africa, and Australasia, 2002–2006. Avian Dis. 2007, 51, 161–166, doi:10.1637/7602-041306r.1.
- Wong, S.S.; Yuen, K.Y. Avian influenza virus infections in humans. Chest 2006, 129, 156–168, doi:10.1378/chest.129.1.156.
- Herfst, S.; Imai, M.; Kawaoka, Y.; Fouchier, R.A. Avian influenza virus transmission to mammals. Curr. Top. Microbiol. Immunol. 2014, 385, 137–155, doi:10.1007/82_2014_387.
- Ghedin, E.; Sengamalay, N.A.; Shumway, M.; Zaborsky, J.; Feldblyum, T.; Subbu, V.; Spiro, D.J.; Sitz, J.; Koo, H.; Bolotov, P.; et al. Large-scale sequencing of human influenza reveals the dynamic nature of viral genome evolution. Nature 2005, 437, 1162–1166, doi:10.1038/nature04239.
- Dou, D.; Revol, R.; Östbye, H.; Wang, H.; Daniels, R. Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement. Front. Immunol. 2018, 9, 1581, doi:10.3389/fimmu.2018.01581.
- Jagger, B.W.; Wise, H.M.; Kash, J.C.; Walters, K.A.; Wills, N.M.; Xiao, Y.L.; Dunfee, R.L.; Schwartzman, L.M.; Ozinsky, A.; Bell, G.L.; et al. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science 2012, 337, 199–204, doi:10.1126/science.1222213.
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179.
- Fouchier, R.A.; Munster, V.; Wallensten, A.; Bestebroer, T.M.; Herfst, S.; Smith, D.; Rimmelzwaan, G.F.; Olsen, B.; Osterhaus, A.D. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 2005, 79, 2814–2822, doi:10.1128/jvi.79.5.2814-2822.2005.
- OIE. Influenza A Cleavage Site. World Organization for Animal Health (OIE): Paris, France, 2014. Available online: https://www.oie.int/doc/ged/D13484.PDF (accessed on 2 May 2020).
- Böttcher-Friebertshäuser, E.; Garten, W.; Matrosovich, M.; Klenk, H.D. The hemagglutinin: A determinant of pathogenicity. Curr. Top. Microbiol. 2014, 385, 3–34, doi:10.1007/82_2014_384.
- Alexander, D.J. A review of avian influenza in different bird species. Vet. Microbiol. 2000, 74, 3–13, doi:10.1016/s0378-1135(00)00160-7.
- Guo, Y.J.; Krauss, S.; Senne, D.A.; Mo, I.P.; Lo, K.S.; Xiong, X.P.; Norwood, M.; Shortridge, K.F.; Webster, R.G.; Guan, Y. Characterization of the pathogenicity of members of the newly established H9N2 influenza virus lineages in Asia. Virology 2000, 267, 279–288, doi:10.1006/viro.1999.0115.
- Guan, Y.; Shortridge, K.F.; Krauss, S.; Webster, R.G. Molecular characterization of H9N2 influenza viruses: Were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc. Natl. Acad. Sci. USA 1999, 96, 9363–9367, doi:10.1073/pnas.96.16.9363.
- Suarez, D.L.; Perdue, M.L.; Cox, N.; Rowe, T.; Bender, C.; Huang, J.; Swayne, D.E. Comparisons of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Kong. J. Virol. 1998, 72, 6678–6688.
- Lam, T.T.; Wang, J.; Shen, Y.; Zhou, B.; Duan, L.; Cheung, C.L.; Ma, C.; Lycett, S.J.; Leung, C.Y.; Chen, X.; et al. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature 2013, 502, 241–244, doi:10.1038/nature12515.
- Smith, G.J.; Donis, R.O. Nomenclature updates resulting from the evolution of avian influenza A(H5) virus clades 2.1.3.2a, 2.2.1, and 2.3.4 during 2013–2014. Influenza Other Respir. Viruses 2015, 9, 271–276, doi:10.1111/irv.12324.
- Pusch, E.A.; Suarez, D.L. The Multifaceted Zoonotic Risk of H9N2 Avian Influenza. Vet. Sci. 2018, 5, 82, doi:10.3390/vetsci5040082.
- Zhuang, Q.; Wang, S.; Liu, S.; Hou, G.; Li, J.; Jiang, W.; Wang, K.; Peng, C.; Liu, D.; Guo, A.; et al. Diversity and distribution of type A influenza viruses: An updated panorama analysis based on protein sequences. Virol. J. 2019, 16, 85, doi:10.1186/s12985-019-1188-7.
- Ahmed, S.S.; Ersbøll, A.K.; Biswas, P.K.; Christensen, J.P.; Hannan, A.S.; Toft, N. Ecological determinants of highly pathogenic avian influenza (H5N1) outbreaks in Bangladesh. PLoS ONE 2012, 7, e33938, doi:10.1371/journal.pone.0033938.
- Biswas, P.K.; Christensen, J.P.; Ahmed, S.S.; Barua, H.; Das, A.; Rahman, M.H.; Giasuddin, M.; Hannan, A.S.; Habib, M.A.; Ahad, A.; et al. Avian influenza outbreaks in chickens, Bangladesh. Emerg. Infect. Dis. 2008, 14, 1909–1912, doi:10.3201/eid1412.071567.
- Barman, S.; Marinova-Petkova, A.; Hasan, M.K.; Akhtar, S.; El-Shesheny, R.; Turner, J.C.; Franks, J.; Walker, D.; Seiler, J.; Friedman, K.; et al. Role of domestic ducks in the emergence of a new genotype of highly pathogenic H5N1 avian influenza A viruses in Bangladesh. Emerg. Microbes Infect. 2017, 6, e72, doi:10.1038/emi.2017.60.
- Yang, G.; Chowdury, S.; Hodges, E.; Rahman, M.Z.; Jang, Y.; Hossain, M.E.; Jones, J.; Stark, T.J.; Di, H.; Cook, P.W.; et al. Detection of highly pathogenic avian influenza A(H5N6) viruses in waterfowl in Bangladesh. Virology 2019, 534, 36–44, doi:10.1016/j.virol.2019.05.011.
- Jannat, N.; Chowdhury, E.H.; Parvin, R.; Begum, J.A.; Khan, M.A.H.N.A.; Islam, M.R. Investigation of an outbreak of low pathogenic avian influenza in poultry in Bangladesh. Int. J. Livest. Res. 2013, 3, 21–32.
- Parvin, R.; Heenemann, K.; Halami, M.Y.; Chowdhury, E.H.; Islam, M.R.; Vahlenkamp, T.W. Full-genome analysis of avian influenza virus H9N2 from Bangladesh reveals internal gene reassortments with two distinct highly pathogenic avian influenza viruses. Arch. Virol. 2014, 159, 1651–1661, doi:10.1007/s00705-014-1976-8.
- Sarker, R.D.; Giasuddin, M.; Chowdhury, E.H.; Islam, M.R. Serological and virological surveillance of avian influenza virus in domestic ducks of the north-east region of Bangladesh. BMC Vet. Res. 2017, 13, 180, doi:10.1186/s12917-017-1104-6.
- Rimi, N.A.; Hassan, M.Z.; Chowdhury, S.; Rahman, M.; Sultana, R.; Biswas, P.K.; Debnath, N.C.; Islam, S.S.; Ross, A.G. A Decade of Avian Influenza in Bangladesh: Where Are We Now? Trop. Med. Infect. Dis. 2019, 4, 119, doi:10.3390/tropicalmed4030119.
- Khatun, A.; Giasuddin, M.; Islam, K.M.; Khanom, S.; Samad, M.A.; Islam, M.R.; Noor, M.; Bhuiyan, J.U.; Kim, W.I.; Eo, S.K.; et al. Surveillance of avian influenza virus type A in semi-scavenging ducks in Bangladesh. BMC Vet. Res. 2013, 9, 196, doi:10.1186/1746-6148-9-196.
- Parvin, R.; Kabiraj, C.K.; Mumu, T.T.; Chowdhury, E.H.; Islam, M.R.; Beer, M.; Harder, T. Active virological surveillance in backyard ducks in Bangladesh: Detection of avian influenza and gammacoronaviruses. Avian Pathol. 2020, 1–8, doi:10.1080/03079457.2020.1753654.
- Parvin, R.; Kamal, A.H.; Haque, M.E.; Chowdhury, E.H.; Giasuddin, M.; Islam, M.R.; Vahlenkamp, T.W. Genetic characterization of highly pathogenic H5N1 avian influenza virus from live migratory birds in Bangladesh. Virus Genes 2014, 49, 438–448.
- Takekawa, J.Y.; Prosser, D.J.; Collins, B.M.; Douglas, D.C.; Perry, W.M.; Yan, B.; Ze, L.; Hou, Y.; Lei, F.; Li, T.; et al. Movements of wild ruddy shelducks in the Central Asian Flyway and their spatial relationship to outbreaks of highly pathogenic avian influenza H5N1. Viruses 2013, 5, 2129–2152, doi:10.3390/v5092129.
- Palm, E.C.; Newman, S.H.; Prosser, D.J.; Xiao, X.; Ze, L.; Batbayar, N.; Balachandran, S.; Takekawa, J.Y. Mapping migratory flyways in Asia using dynamic Brownian bridge movement models. Mov. Ecol. 2015, 3, 3, doi:10.1186/s40462-015-0029-6.
- Lepage, D. Avibase. 2015. Available online: http://avibase.bsc-eoc.org/checklist.jsp?lang=EN®ion=bd&list=clements (accessed on 12 May 2020).
- Marinova-Petkova, A.; Shanmuganatham, K.; Feeroz, M.M.; Jones-Engel, L.; Hasan, M.K.; Akhtar, S.; Turner, J.; Walker, D.; Seiler, P.; Franks, J.; et al. The Continuing Evolution of H5N1 and H9N2 Influenza Viruses in Bangladesh Between 2013 and 2014. Avian Dis. 2016, 60, 108–117, doi:10.1637/11136-050815-Reg.
- Shanmuganatham, K.; Feeroz, M.M.; Jones-Engel, L.; Smith, G.J.; Fourment, M.; Walker, D.; McClenaghan, L.; Alam, S.M.; Hasan, M.K.; Seiler, P.; et al. Antigenic and molecular characterization of avian influenza A(H9N2) viruses, Bangladesh. Emerg. Infect. Dis. 2013, 19, 1393–1402, doi:10.3201/eid1909.130336.
- Gerloff, N.A.; Khan, S.U.; Zanders, N.; Balish, A.; Haider, N.; Islam, A.; Chowdhury, S.; Rahman, M.Z.; Haque, A.; Hosseini, P.; et al. Genetically Diverse Low Pathogenicity Avian Influenza A Virus Subtypes Co-Circulate among Poultry in Bangladesh. PLoS ONE 2016, 11, e0152131, doi:10.1371/journal.pone.0152131.
- Khan, S.U.; Gurley, E.S.; Gerloff, N. Avian influenza surveillance in domestic waterfowl and environment of live bird markets in Bangladesh, 2007–2012. Sci. Rep. 2018, 8, 9396, doi:10.1038/s41598-018-27515-w.
- Dolberg, F. Poultry Sector Country Overview: Bangladesh. FAO Animal Production and Health Division. 2008. Available online: http://www.fao.org/3/a-ai319e.pdf (accessed on 2 May 2020).
- The World Bank. Agriculture & Rural Development. 2013. Available online: http://data.worldbank.org/topic/agriculture-and-rural-development?display=graph (accessed on 12 April 2020).
- Sultana, R.; Nahar, N.; Rimi, N.A.; Azad, S.; Islam, M.S.; Gurley, E.S.; Luby, S.P. Backyard poultry raising in Bangladesh: A valued resource for the villagers and a setting for zoonotic transmission of avian influenza. A qualitative study. Rural Remote Health 2012, 12, 1927.
- FAO. Comparative Performance of Sonali Chickens, Commercial Broilers, Layersand Local Non-descript (deshi) Chickens in Selected Areas of Bangladesh; Food and Agriculture Organization (FAO): Rome, Italy, 2015. Available online: www.fao.org/3/a-i4725e.pdf (accessed on 10 April 2020).
- Bhuiyan, A.K.F.H.; Bhuiyan, M.S.A.; Deb, G.K. Indigenous chicken genetic resources in Bangladesh: Current status and future outlook. Anim. Genet. Resour. Inf. 2005, 36, 73–84, doi:10.1017/S1014233900001899.
- Biswas, P.K.; Christensen, J.P.; Ahmed, S.S.; Barua, H.; Das, A.; Rahman, M.H.; Giasuddin, M.; Hannan, A.S.; Habib, A.M.; Debnath, N.C. Risk factors for infection with highly pathogenic influenza A virus (H5N1) in commercial chickens in Bangladesh. Vet. Rec. 2009, 164, 743–746, doi:10.1136/vr.164.24.743.
- Biswas, P.K.; Christensen, J.P.; Ahmed, S.S.; Das, A.; Rahman, M.H.; Barua, H.; Giasuddin, M.; Hannan, A.S.; Habib, M.A.; Debnath, N.C. Risk for infection with highly pathogenic avian influenza virus (H5N1) in backyard chickens, Bangladesh. Emerg. Infect. Dis. 2009, 15, 1931–1936, doi:10.3201/eid1512.090643.
- Huque, K.; Khan, M. Socio-geographic distribution of livestock and poultry in Bangladesh-A review. Bangladesh J. Anim. Sci. 2017, 46, 65–81, doi:10.3329/bjas.v46i1.32180.
- DLS. DLS. Livestock Economy at a Glance, Government of Bangladesh. Department of Livestock Services (DLS), Bangladesh. 2018. Available online: http://dls.portal.gov.bd/sites/default/files/files/dls.portal.gov.bd/page/ee5f4621_fa3a_40ac_8bd9_898fb8ee4700/Livestock%20Economy%20at%20a%20glance%20%20%282017–2018%29.pdf (accessed on 24 April, 2020).
- Parvin, R.; Begum, J.A.; Nooruzzaman, M.; Chowdhury, E.H.; Islam, M.R.; Vahlenkamp, T.W. Review analysis and impact of co-circulating H5N1 and H9N2 avian influenza viruses in Bangladesh. Epidemiol. Infect. 2018, 146, 1259–1266, doi:10.1017/S0950268818001292.
- Alam, J.; Giasuddin, M.; Samad, M.A.; Taimur, M.J.F.A. Recent evidence of Avian Influenza in Bangladesh: A review. World Poult. Sci. J. 2010, 66, 455–464, doi:10.1017/S004393391000053X.
- Kim, S.H. Challenge for One Health: Co-Circulation of Zoonotic H5N1 and H9N2 Avian Influenza Viruses in Egypt. Viruses 2018, 10, 121, doi:10.3390/v10030121.
- Turner, J.C.; Feeroz, M.M.; Hasan, M.K.; Akhtar, S.; Walker, D.; Seiler, P.; Barman, S.; Franks, J.; Jones-Engel, L.; McKenzie, P.; et al. Insight into live bird markets of Bangladesh: An overview of the dynamics of transmission of H5N1 and H9N2 avian influenza viruses. Emerg. Microbes Infect. 2017, 6, e12, doi:10.1038/emi.2016.142.
- Moyen, N.; Ahmed, G.; Gupta, S.; Tenzin, T.; Khan, R.; Khan, T.; Debnath, N.; Yamage, M.; Pfeiffer, D.U.; Fournie, G. A large-scale study of a poultry trading network in Bangladesh: Implications for control and surveillance of avian influenza viruses. BMC Vet. Res. 2018, 14, 12, doi:10.1186/s12917-018-1331-5.
- Nasreen, S.; Khan, S.U.; Luby, S.P.; Gurley, E.S.; Abedin, J.; Zaman, R.U.; Sohel, B.M.; Rahman, M.; Hancock, K.; Levine, M.Z.; et al. Highly pathogenic Avian Influenza A(H5N1) virus infection among workers at live bird markets, Bangladesh, 2009–2010. Emerg. Infect. Dis. 2015, 21, 629–637, doi:10.3201/eid2104.141281.
- FAO. Approaches to Controlling, Preventing and Eliminating H5N1 Highly Pathogenic Avian Influenza in Endemic Countries. 2011. Available online: http://www.fao.org/3/i2150e/i2150e00.htm (accessed on 12 March 2020).
- Killingley, B.; Nguyen-Van-Tam, J. Routes of influenza transmission. Influenza Other Respir. Viruses 2013, 7, 42–51, doi:10.1111/irv.12080.
- Peacock, T.H.P.; James, J.; Sealy, J.E.; Iqbal, M. A Global Perspective on H9N2 Avian Influenza Virus. Viruses 2019, 11, 620, doi:10.3390/v11070620.
- Parvin, , R.; Begum, J.A.; Chowdhury, E.H.; Islam, M.R. Co-subsistence of avian influenza virus subtypes of low and high pathogenicity in Bangladesh: Challenges for diagnosis, risk assessment and control. Sci. Rep. 2019, 9, 8306, doi:10.1038/s41598-019-44220-4.
- Rimi, N.A.; Sultana, R.; Muhsina, M.; Uddin, B.; Haider, N.; Nahar, N.; Zeidner, N.; Sturm-Ramirez, K.; Luby, S.P. Biosecurity Conditions in Small Commercial Chicken Farms, Bangladesh 2011–2012. Ecohealth 2017, 14, 244–258, doi:10.1007/s10393-017-1224-2.
- Sarker, B.C.; Alam, M.A.; Rahman, M.M.; Islam, A.F.M.T.; Chowdhury, M.G.F. Waste management of commercial poultry farms in Bangladesh. J. Innov. Dev. Strateg. 2009, 3, 34–37.
- Biswas, P.K.; Giasuddin, M.; Nath, B.K.; Islam, M.Z.; Debnath, N.C.; Yamage, M. Biosecurity and Circulation of Influenza A (H5N1) Virus in Live-Bird Markets in Bangladesh, 2012. Transbound. Emerg. Dis. 2017, 64, 883–891, doi:10.1111/tbed.12454.
- Khan, S.U.; Berman, L.; Haider, N.; Gerloff, N.; Rahman, M.Z.; Shu, B.; Rahman, M.; Dey, T.K.; Davis, T.C.; Das, B.C.; et al. Investigating a crow die-off in January-February 2011 during the introduction of a new clade of highly pathogenic avian influenza virus H5N1 into Bangladesh. Arch. Virol. 2014, 159, 509–518, doi:10.1007/s00705-013-1842-0.
- SAPPLPP. Combating Bird Flu through Bio-security Measures at Farm and Community Level: Evidence from Bangladesh; Good Practice Note: Delhi, India, 2010. Available online: http://sapplpp.org/publications/good-practice-notes-briefs/small-holder-poultry/BDGP03-combating-bird-flu-through-bio-security-measures.html#.XvRGsigzbIU (accessed on 24 March 2020).
- Sultana, R.; Rimi, N.A.; Azad, S.; Islam, M.S.; Khan, M.S.; Gurley, E.S.; Nahar, N.; Luby, S.P. Bangladeshi backyard poultry raisers’ perceptions and practices related to zoonotic transmission of avian influenza. J. Infect. Dev. Countr. 2012, 6, 156–165, doi:10.3855/jidc.2242.
- Rimi, N.A.; Sultana, R.; Ishtiak-Ahmed, K.; Rahman, M.Z.; Hasin, M.; Islam, M.S.; Azziz-Baumgartner, E.; Nahar, N.; Gurley, E.S.; Luby, S.P. Understanding the failure of a behavior change intervention to reduce risk behaviors for avian influenza transmission among backyard poultry raisers in rural Bangladesh: A focused ethnography. Bmc Public Health 2016, 16, 858, doi:10.1186/s12889-016-3543-6.
- Popy, F.Y.; Chowdhury, Q.; Alam, S.; Roy, S.; Dipta, P.M.; Ahmed, J. Backyard Poultry Management and Production System at Barlekha Upazila, Moulvibazar, Bangladesh. Int. J. Sci. Bus. 2018, 2, 90–100.
- Conan, A.; Goutard, F.L.; Sorn, S.; Vong, S. Biosecurity measures for backyard poultry in developing countries: A systematic review. BMC Vet. Res. 2012, 8, 240, doi:10.1186/1746-6148-8-240.
- Shanta, , I.S.; Hasnat, M.A.; Zeidner, N.; Gurley, E.S.; Azziz-Baumgartner, E.; Sharker, M.A.Y.; Hossain, K.; Khan, S.U.; Haider, N. Raising Backyard Poultry in Rural Bangladesh: Financial and Nutritional Benefits, but Persistent Risky Practices. Transbound. Emerg. Dis. 2017, 64, 1454–1464, doi:10.1111/tbed.12536.
- Rimi, N.A.; Sultana, R.; Ishtiak-Ahmed, K.; Haider, N.; Azziz-Baumgartner, E.; Nahar, N.; Luby, S.P. Where backyard poultry raisers seek care for sick poultry: Implications for avian influenza prevention in Bangladesh. Bmc Public Health 2018, 18, 969, doi:10.1186/s12889-018-5819-5.
- Ibrahim, N.; Akhter, M.; Mamun, S.; Chowdhury, E.H.; Das, P.M. Bio-security in small scale poultry farms against avian influenza: Knowledge, attitude and practices. Asian J. Med. Biol. Res. 2016, 1, 670–676, doi:10.3329/ajmbr.v1i3.26495.
- Islam, M.; Huque, Q. Practices of bio-security in small-scale broiler farms. Bangladesh Vet. 2007, 24, 72–78.
- Osmani, M.G.; Ward, M.P.; Giasuddin, M.; Islam, M.R.; Kalam, A. The spread of highly pathogenic avian influenza (subtype H5N1) clades in Bangladesh, 2010 and 2011. Prev. Vet. Med. 2014, 114, 21–27, doi:10.1016/j.prevetmed.2014.01.010.
- Mondal, S.P.; Tardif-Douglin, D.; Ryan-Silva, R.; Magnani, R. Controlling highly pathogenic avian influenza, Bangladesh. Emerg. Infect. Dis. 2012, 18, 2083–2085, doi:10.3201/eid1812.120635.
- Sayeed, M.A.; Smallwood, C.; Imam, T.; Mahmud, R.; Hasan, R.B.; Hasan, M.; Anwer, M.S.; Rashid, M.H.; Hoque, M.A. Assessment of hygienic conditions of live bird markets on avian influenza in Chittagong metro, Bangladesh. Prev. Vet. Med. 2017, 142, 7–15, doi:10.1016/j.prevetmed.2017.04.009.
