Chitin, being the second most abundant biopolymer after next to cellulose, has been gaining popularity since its initial first discovery by Braconot in 1811. However, fundamental knowledge and literature on about chitin and its derivatives from insects are difficult to obtain. The most common and sought-after sources of chitin are shellfishes (especially crustaceans) and other aquatic invertebrates. The amount of shellfishes available is obviously restricted by the amount of food waste that is allowed; hence, it is a limited resource. Therefore,Hence, insects are the best choices since, out of 1.3 million species in the world, 900,000 are insects, making them the most abundant species in the world. In this review, a total of 82 samples from shellfishes—crustaceans and mollusks (n = 46), insects (n = 23), and others (n = 13)—have been collected and studied for their chemical extraction of chitin and its derivatives. The aim of this paper is to review the extraction method of chitin and chitosan for a comparison of the optimal demineralization and deproteinization processes with, so as to consideration of insects as alternative sources of chitin. The methods employed in this review are based on comprehensive bibliographic research. Based on previous data, chitin and chitosan yield contents of insects in their study favorably compares and competes with those of commercial chitin and chitosan—for example, 45% in Bombyx eri, 36.6% in Periostracum cicadae (cicada sloughs), and 26.2% in Chyrysomya megacephala. Therefore, according to the data reported by previous researchers, with comparable yield values to those of against crustacean chitin and the great interests of in insects as alternative sources, efforts towards comprehensive knowledge in this field are relevant.
- chitin
- chitosan
- chemical extraction
- deproteinization
- demineralization
1. Introduction
Chitin and its derivatives represent a well-reviewed biopolymer with many beneficial applications. The preparations for chitin and its derivatives as a biomaterial vary according to process conditions and potential applications. However, their main sources are crustaceans, and research on alternative sources is still developing. Chitin is the basic structure and a major constituent of the cell wall of many fungi, insect exoskeletons, and crustacean shells. It largely exists in waste from the processing of marine food products, such as shrimp, prawn, crab, lobster, crayfish, squid, cuttlefish, and barnacles [1]. Chitin extraction has been well-known since its first isolation in 1811 by Henri Braconnot from some of the higher fungi, and chitin is the earliest known polysaccharide [2]. On the other hand, Albert Hoffman was the first researcher to determine the structure of chitin [3]. His main interest was the chemistry of plants and animals, and he later conducted important research during his study on the chemical structure of common animal substances and hence the discovery of chitin, for which he received his doctorate, with distinction, in the spring of 1929 [4].
Chitin is a biopolymer and is the most abundant biopolymer after cellulose, with a production of approximately 10
10
12 tons annually [5][6][7]. Chitin has the same chemical structure as cellulose— a plant fiber [2]. It is a linear polysaccharide composed of β-1,4 linked with N-acetylglucosamine (GlcNAc) units (to be precise, 2-(acetylamino)-2-deoxy-D-glucose) and occurs naturally in three polymorphic forms, with different orientations of the microfibrils, known as α, β, and γ chitin [5][6].
Chitin isolates from crustaceans, other aquatic invertebrates, and arthropods, especially those with a hard exterior, usually come in the α-form. This is because the chains are aligned in an anti-parallel structure, thus making the structure more stable owing to stronger and rigid hydrogen bonding. Meanwhile, β-form chains are arranged in a parallel fashion, and most chitin sources with this β-form are obtained from mollusks, such as squid pens [8]. The γ-form contains two parallel strands and one anti-parallel strand of chitin and is found in cocoons of insects. Conversion from the β-form to the α-form is possible, but not the reverse. It has been reported that the isolation of chitin from different sources is affected by the source and the percentage of chitin present in the source, and it was found that the crystallinity, purity, and polymer chain arrangement vary, according to the source. Crab, shrimp, and crayfish have been preferred for the commercial production of chitin, but new alternative chitin sources, such as fungus and insects, could be exploited [9][10][11].
Chitin isolates from crustaceans, other aquatic invertebrates, and arthropods, especially those with a hard exterior, usually come in the α-form. This is because the chains are aligned in an anti-parallel structure, thus making the structure more stable owing to stronger and rigid hydrogen bonding. Meanwhile, β-form chains are arranged in a parallel fashion, and most chitin sources with this β-form are obtained from mollusks, such as squid pens [8]. The γ-form contains two parallel strands and one anti-parallel strand of chitin and is found in cocoons of insects. Conversion from the β-form to the α-form is possible, but not the reverse. It has been reported that the isolation of chitin from different sources is affected by the source and the percentage of chitin present in the source, and it was found that the crystallinity, purity, and polymer chain arrangement vary, according to the source. Crab, shrimp, and crayfish have been preferred for the commercial production of chitin, but new alternative chitin sources, such as fungus and insects, could be exploited [9,10,11].Chitosan is derived from chitin by removing a sufficient number of acetyl groups (CH
Chitosan is derived from chitin by removing a sufficient number of acetyl groups (CH3
–CO) for the molecule to be soluble in most diluted acids. Chitosan is a fiber-like substance and a homopolymer of β-(1→4)-linked N-acetyl-D-glucosamine. The actual difference between chitin and chitosan is the acetyl content of the polymer. Chitosan, which has a free amino group, is the most useful derivative of chitin [12]. Chitosan is a modified natural carbohydrate polymer that has been found in a wide range of natural sources, such as crustaceans, mollusks, fungi, insects, and some algae [13]. Chitosan is a non-toxic, biodegradable polymer of a high molecular weight and is very similar to cellulose in terms of its chemical structure. The only difference between chitosan and cellulose is the amine (-NH2) group in the C-2 position of chitosan instead of the hydroxyl (-OH) group found in cellulose. However, unlike plant fiber, chitosan possesses positive ionic charges that give it the ability to chemically bind with negatively charged fats, lipids, cholesterol, metal ions, proteins, and macromolecules. In this respect, chitin and chitosan have attained increasing commercial interest as suitable materials due to their excellent properties, including their biocompatibility, biodegradability, adsorption, and abilities to form films and chelate metal ions [14][15][16].
) group in the C-2 position of chitosan instead of the hydroxyl (-OH) group found in cellulose. However, unlike plant fiber, chitosan possesses positive ionic charges that give it the ability to chemically bind with negatively charged fats, lipids, cholesterol, metal ions, proteins, and macromolecules. In this respect, chitin and chitosan have attained increasing commercial interest as suitable materials due to their excellent properties, including their biocompatibility, biodegradability, adsorption, and abilities to form films and chelate metal ions [14,15,16].Figure 1a indicates the chemical configuration of chitin. Figure 1b shows the structure of the chitin molecule, and presents two of the N-acetylglucosamine units that repeat to form long chains in the β-(1→4)-linkage. These units form covalent β-(1→4)-linkages (similar to the linkages between glucose units forming cellulose). Therefore, chitin may be described as cellulose with one hydroxyl group on each monomer replaced with an acetyl amine group. This allows for increased hydrogen bonding between adjacent polymers, giving the chitin–polymer matrix an increased strength [1][12][1,12,17].
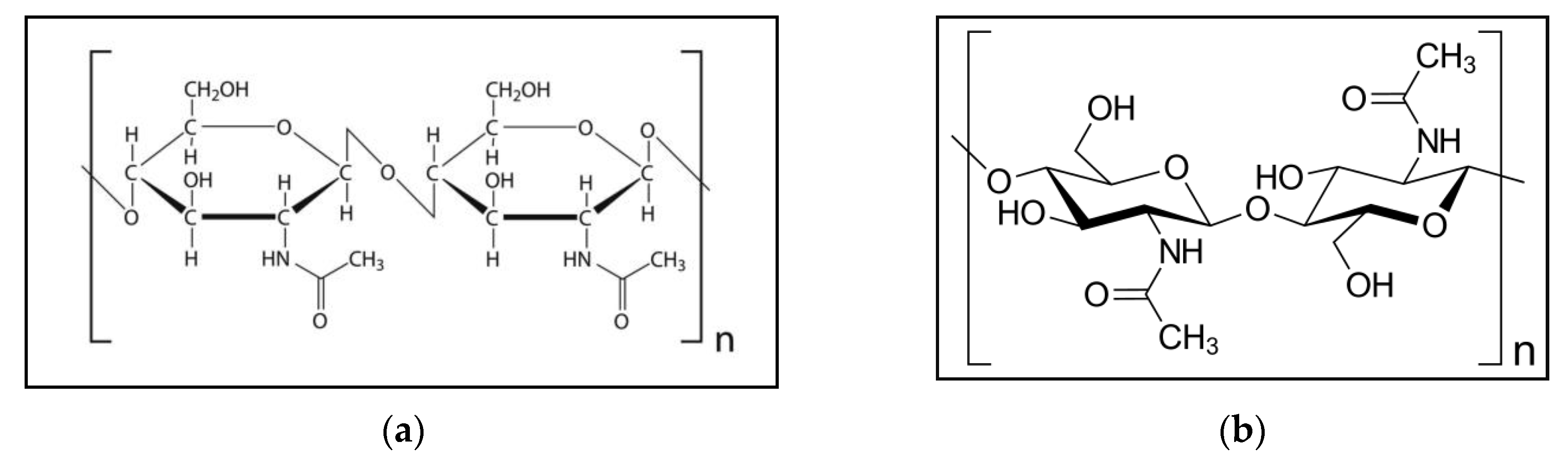
Figure 1. (a) Chemical configuration of chitin; (b) structure of the chitin molecule, showing two of the N-acetylglucosamine units that repeat to form long chains in the β-(1→4)-linkage.
In its pure, unmodified form, chitin is translucent, pliable, resilient, and quite tough. In most arthropods, however, it is often modified, occurring largely as a component of composite materials, such as in sclerotin, a tanned proteinaceous matrix, which forms much of the exoskeleton of insects. Combined with calcium carbonate (CaCO
3
3. Another difference between pure and composite forms can be seen by comparing the flexible body wall of a caterpillar (mainly chitin) to the stiff, light elytron of a beetle (containing a large proportion of sclerotin) [14][15][16][18].
In addition, chitin and chitosan have the ability to bond with different links, forming materials such as fibers, hydrogels, beads, sponges, and membranes. Chitosan has been used in several fields, such as agriculture, food protection [19], biomedicine [20], cosmeceuticals [21], and pharmaceuticals [22] as drug delivery systems or in drug formulations [15][16][18][23][24][25][26][27]. Sources of chitin and chitosan that are used in such beneficial applications mostly consist of shellfish, mainly crustaceans [28][29]. However, interest in chitin and chitosan from insects as alternative sources other than crustaceans has increased steadily over the last several decades. A handful of researchers have paid attention to and elucidated the process of extraction, optimization, and yield, as well as the characterization of chitin and chitosan from various sources, especially insects. Hence, insects are gaining popularity as more researchers study them. They represent the best choice as an alternative since out of 1.3 million species in the world, 900,000 are insects, making them the most abundant species in the world [30][31]. Therefore, the aim of this paper was to review the extraction method of chitin and chitosan for a comparison of the optimal demineralization and deproteinization processes, with a consideration of insects as alternative sources of chitin.
Even though chitin and chitosan have been called our “last biomass resource” and are expected to lead to a new functional polymer, their utilization is scarce, and they have hardly been explored. Though a variety of interesting biological activities have been reported throughout the years, practical application has lagged. One of the main reasons for this is that these biological activities are not specific to chitosan; such activities are also found in other materials. The second reason is the issue of cost, since chitosan is relatively expensive (20–30 US dollars per kg). If a specific biological activity was found to be unique to chitosan materials, practical utilization would be encouraged despite the cost, especially for biomedical use. Chitin and chitosan are structurally similar to heparin, chondroitin sulfate, and hyaluronic acid, which are all biologically important mucopolysaccharides in all mammals. These mucopolysaccharides are anionic polymers owing to substituent carboxyl and sulfuryl groups. On the other hand, chitosan is almost the only cationic polysaccharide in nature, and it is nontoxic and biodegradable in the human body [6]. This special property is worth noting in regard to biomedical applications [32]. However, since chitosan does not dissolve in neutral and basic aqueous media, its biomedical use is limited. The chemical modification of chitosan provides derivatives that are soluble at a neutral and basic pH. Moreover, chemical modification can be used to attach various functional groups and to control hydrophobic, cationic, and anionic properties [33][34]. Further studies and the development of chitin, chitosan, and their derivatives for use in applied biomaterials need to be carried out [5]. These points can be considered in future developments in the field of biomaterials from insects.
2. Extraction of Chitin
Chitin is gaining popularity, especially because many beneficial traits are being discovered for fertilizers [35][36][37], food additives [38], emulsifying agents [39][40], and surgical [41][42][43] and medicinal [44] applications, as well as in agricultural [45], pharmaceutical [46][47], and even cosmeceutical [21][48][49][50] fields. The most common extraction methods are biological [51][52][53][54] and chemical [10][17] treatments. As mentioned above, chemical treatment involves two major steps with an optional treatment. Demineralization is an acidic step that removes minerals associated with the basic structure of the exoskeleton, and deproteinization is a basic step that removes the proteins bound together with all constituents. Generally, for comparison, the parameters for acidic treatment (concentration, temperature, time, and solution-to-solid ratio) used for chemical extraction from insects are moderate compared to crustaceans’ chitin isolation requirements. This is because insects have lower levels of inorganic material (less than 10%) compared to crustacean shells (20%–40%) [13].
Chitin is gaining popularity, especially because many beneficial traits are being discovered for fertilizers [35,36,37], food additives [38], emulsifying agents [39,40], and surgical [41,42,43] and medicinal [44] applications, as well as in agricultural [45], pharmaceutical [46,47], and even cosmeceutical [21,48,49,50] fields. The most common extraction methods are biological [51,52,53,54] and chemical [10,17] treatments. As mentioned above, chemical treatment involves two major steps with an optional treatment. Demineralization is an acidic step that removes minerals associated with the basic structure of the exoskeleton, and deproteinization is a basic step that removes the proteins bound together with all constituents. Generally, for comparison, the parameters for acidic treatment (concentration, temperature, time, and solution-to-solid ratio) used for chemical extraction from insects are moderate compared to crustaceans’ chitin isolation requirements. This is because insects have lower levels of inorganic material (less than 10%) compared to crustacean shells (20%–40%) [13].There are numerous alternative methods of extraction for chitin, where their sole purpose is to remove impurities and foreign organic matter, including protein and minerals, because it is naturally formed in the structure of the exoskeleton [10][55]. However, detrimental effects on the molecular weight and the degree of acetylation are unavoidable with any of the extraction processes.
There are numerous alternative methods of extraction for chitin, where their sole purpose is to remove impurities and foreign organic matter, including protein and minerals, because it is naturally formed in the structure of the exoskeleton [10,55]. However, detrimental effects on the molecular weight and the degree of acetylation are unavoidable with any of the extraction processes.3. Conclusions
The chitin and chitosan contents of insects in the studies examined in this entry favorably compare and compete with those of commercial chitin and chitosan. The characteristics of chitin and its derivatives from insects are similar to those of commercial chitin from crustaceans and other aquatic invertebrates. It is also non-toxic and very safe to use. In addition, because of their large numbers and the ease of breeding, insects provide an abundant resource for larger-scale chitin production. However, the mechanism and applications for chitin and chitosan from insects are still limited. To elucidate this insufficient information, further studies are needed. Therefore, according to the data reported by previous researchers, showing comparable yield values to those of crustacean chitin, and owing to the great interest in insects as an alternative source of chitin, efforts towards comprehensive knowledge in this field are of merit.
