Anti-Gal are the most abundant xenoreactive natural antibodies. They are supposed to stem from immunization against the gut microbiota and have been implicated in the pathogenesis of several diseases, including multiple sclerosis.
- alpha-gal epitope
- Alzheimer’s disease
- microbiota
1. Introduction
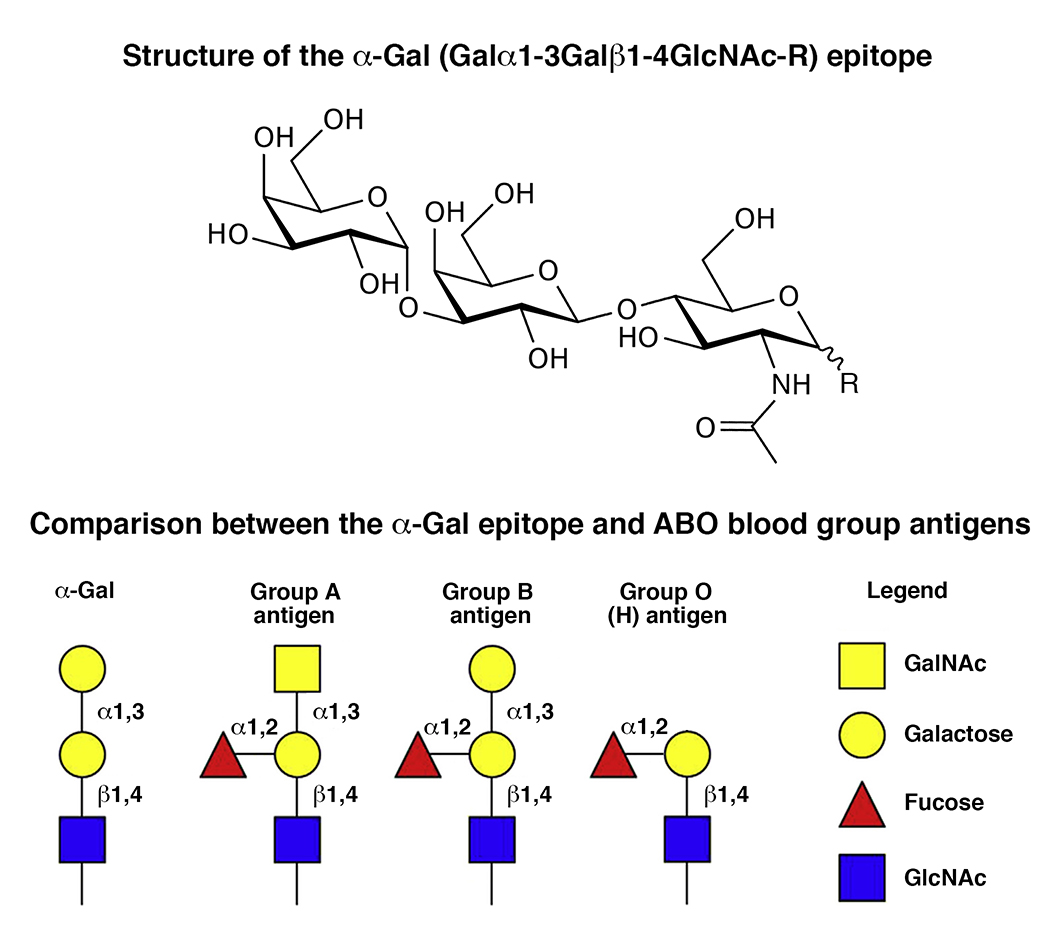
2. Altered Blood Levels of Anti-Gal Antibodies in Alzheimer’s Disease
3. Conclusions
Anti-Gal antibodies are the most abundant xenoreactive natural antibodies. These antibodies target the alpha-Gal epitope, terminally located in the oigosaccharide units of glycoproteins or glycolipids of all mammals except apes, Old World monkeys and humans. The alpha-Gal epitope is constitutively expressed in several bacteria constituting the brain microbiota, and alpha-Gal-like epitopes have been detected in the gray matter, amyloid deposits, neurofibrillary tangles and corpora amylacea in the human brain, suggesting a potential link between anti-Gal and Alzheimer’s disease etiopathogenesis.
References
- Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460.
- Fleming, R.; Zeisel, J.; Bennet, K. World Alzheimer Report 2020, Vol I and II; Alzheimer’s Disease International: London, UK, 2020.
- McGrowder, D.A.; Miller, F.; Vaz, K.; Nwokocha, C.; Wilson-Clarke, C.; Anderson-Cross, M.; Brown, J.; Anderson-Jackson, L.; Williams, L.; Latore, L.; et al. Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease: Current Evidence and Future Perspectives. Brain Sci. 2021, 11, 215.
- González-Sanmiguel, J.; Schuh, C.M.A.P.; Muñoz-Montesino, C.; Contreras-Kallens, P.; Aguayo, L.G.; Aguayo, S. Complex Interaction between Resident Microbiota and Misfolded Proteins: Role in Neuroinflammation and Neurodegeneration. Cells 2020, 9, 2476.
- Almeida, I.C.; Ferguson, M.A.; Schenkman, S.; Travassos, L.R. Lytic anti-alpha-galactosyl antibodies from patients with chronic Chagas’ disease recognize novel O-linked oligosaccharides on mucin-like glycosyl-phosphatidylinositol-anchored glycoproteins of Trypanosoma cruzi. Biochem. J. 1994, 304, 793.
- Welsh, R.M.; O’Donnell, C.L.; Reed, D.J.; Rother, R.P. Evaluation of the Galalpha1-3Gal epitope as a host modification factor eliciting natural humoral immunity to enveloped viruses. J. Virol. 1998, 72, 4650–4656.
- Han, W.; Cai, L.; Wu, B.; Li, L.; Xiao, Z.; Cheng, J.; Wang, P.G. The wciN gene encodes an α-1,3-galactosyltransferase involved in the biosynthesis of the capsule repeating unit of Streptococcus pneumoniae serotype 6B. Biochemistry 2012, 51, 5804–5810.
- Huai, G.; Qi, P.; Yang, H.; Wang, Y. Characteristics of α-Gal epitope, anti-Gal antibody, α1,3 galactosyltransferase and its clinical exploitation. Int. J. Mol. Med. 2016, 37, 11–20.
- Reyneveld, G.I.; Savelkoul, H.F.J.; Parmentier, H.K. Current Understanding of Natural Antibodies and Exploring the Possibilities of Modulation Using Veterinary Models. A Review. Front. Immunol. 2020, 11, 2139.
- Galili, U. Discovery of the natural anti-Gal antibody and its past and future relevance to medicine. Xenotransplantation 2013, 20, 138–147.
- Galili, U. Human Natural Antibodies to Mammalian Carbohydrate Antigens as Unsung Heroes Protecting against Past, Present, and Future Viral Infections. Antibodies 2020, 9, 25.
- Galili, U. Significance of the evolutionary α1,3-galactosyltransferase (GGTA1) gene inactivation in preventing extinction of apes and old world monkeys. J. Mol. Evol. 2015, 80, 1–9.
- Nguyen, T.G.; McKelvey, K.J.; March, L.M.; Hunter, D.J.; Xue, M.; Jackson, C.J.; Morris, J.M. Aberrant levels of natural IgM antibodies in osteoarthritis and rheumatoid arthritis patients in comparison to healthy controls. Immunol. Lett. 2016, 170, 27–36.
- Palma, J.; Tokarz-Deptuła, B.; Deptuła, J.; Deptuła, W. Natural antibodies–facts known and unknown. Cent. Eur. J. Immunol. 2018, 43, 466–475.
- Le Berre, L.; Rousse, J.; Gourraud, P.A.; Imbert-Marcille, B.M.; Salama, A.; Evanno, G.; Semana, G.; Nicot, A.; Dugast, E.; Guérif, P.; et al. Decrease of blood anti-α1,3 Galactose Abs levels in multiple sclerosis (MS) and clinically isolated syndrome (CIS) patients. Clin. Immunol. 2017, 180, 128–135.
- Montassier, E.; Berthelot, L.; Soulillou, J.P. Are the decrease in circulating anti-α1,3-Gal IgG and the lower content of galactosyl transferase A1 in the microbiota of patients with multiple sclerosis a novel environmental risk factor for the disease? Mol. Immunol. 2018, 93, 162–165.
- Galili, U.; Anaraki, F.; Thall, A.; Hill-Black, C.; Radic, M. One percent of human circulating B lymphocytes are capable of producing the natural anti-Gal antibody. Blood 1993, 82, 2485–2493.
- Jaison, P.L.; Kannan, V.M.; Geetha, M.; Appukuttan, P. Epitopes recognized by serum anti-α-galactoside antibody are present on brain glycoproteins in man. J. Niosci. 1993, 18, 187–193.
- Nishi, K.; Tanegashima, A.; Yamamoto, Y.; Ushiyama, I.; Ikemoto, K.; Yamasaki, S.; Nishimura, A.; Rand, S.; Brinkmann, B. Utilization of lectin-histochemistry in forensic neuropathology: Lectin staining provides useful information for postmortem diagnosis in forensic neuropathology. Leg. Med. 2003, 5, 117–131.
- Montassier, E.; Al-Ghalith, G.A.; Mathé, C.; Le Bastard, Q.; Douillard, V.; Garnier, A.; Guimon, R.; Raimondeau, B.; Touchefeu, Y.; Duchalais, E.; et al. Distribution of Bacterial α1,3-Galactosyltransferase Genes in the Human Gut Microbiome. Front. Immunol 2020, 10, 3000.
- Westfall, S.; Dinh, D.M.; Pasinetti, G.M. Investigation of Potential Brain Microbiome in Alzheimer’s Disease: Implications of Study Bias. J. Alzheimer’s Dis. 2020, 75, 559–570.
- Burk, C.M.; Beitia, R.; Lund, P.K.; Dellon, E.S. High rate of galactose-alpha-1,3-galactose sensitization in both eosinophilic esophagitis and patients undergoing upper endoscopy. Dis. Esophagus 2016, 29, 558–562.
- Safaie, P.; Ham, M.; Kuang, P.; Mehta, A.S.; Wang, M.; Cheifetz, A.S.; Robson, S.; Lau, D.; Block, T.M.; Moss, A.C. Lectin-reactive anti-α-gal in patients with Crohn’s disease: Correlation with clinical phenotypes. Inflamm. Bowel Dis. 2013, 19, 2796–2800.
- Chinuki, Y.; Morita, E. Alpha-Gal-containing biologics and anaphylaxis. Allergol. Int. 2019, 68, 296–300.
- Naso, F.; Stefanelli, U.; Buratto, E.; Lazzari, G.; Perota, A.; Galli, C.; Gandaglia, A. Alpha-Gal Inactivated Heart Valve Bioprostheses Exhibit an Anti-Calcification Propensity Similar to Knockout Tissues. Tissue Eng. Part A 2017, 23, 1181–1195.
- Celarain, N.; Tomas-Roig, J. Aberrant DNA methylation profile exacerbates inflammation and neurodegeneration in multiple sclerosis patients. J. Neuroinflamm. 2020, 17, 21.
- Mangold, A.; Lebherz, D.; Papay, P.; Liepert, J.; Hlavin, G.; Lichtenberger, C.; Adami, A.; Zimmermann, M.; Klaus, D.; Reinisch, W.; et al. Anti-Gal titers in healthy adults and inflammatory bowel disease patients. Transp. Proc. 2011, 43, 3964–3968.
- Weksler, M.E.; Relkin, N.; Turkenich, R.; La Russe, S.; Zhou, L.; Szabo, P. Patients with Alzheimer disease have lower levels of serum anti-amyloid peptide antibodies than healthy elderly individuals. Exp. Gerontol. 2002, 37, 943–948.
- Britschgi, M.; Olin, C.E.; Johns, H.T.; Takeda-Uchimura, Y.; LeMieux, M.C.; Rufibach, K.; Rajadas, J.; Zhang, H.; Tomooka, B.; Robinson, W.H.; et al. Neuroprotective natural antibodies to assemblies of amyloidogenic peptides decrease with normal aging and advancing Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2009, 106, 12145–12150.
- Heineke, M.H.; van Egmond, M. Immunoglobulin A: Magic bullet or Trojan horse? Eur. J. Clin. Investig. 2017, 47, 184–192.
- Leblhuber, F.; Walli, J.; Tilz, G.P.; Wachter, H.; Fuchs, D. Systemische Veränderungen des Immunsystems bei Patienten mit Alzheimer-Demenz [Systemic changes of the immune system in patients with Alzheimer’s dementia]. Dtsch. Med. Wochenschr. 1998, 123, 787–791.
- de la Rubia Ortí, J.E.; Sancho Castillo, S.; Benlloch, M.; Julián Rochina, M.; Corchón Arreche, S.; García-Pardo, M.P. Impact of the Relationship of Stress and the Immune System in the Appearance of Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 55, 899–903.
- de la Rubia Ortí, J.E.; Prado-Gascó, V.; Sancho Castillo, S.; Julián-Rochina, M.; Romero Gómez, F.J.; García-Pardo, M.P. Cortisol and IgA are Involved in the Progression of Alzheimer’s Disease. A Pilot Study. Cell. Mol. Neurobiol. 2019, 39, 1061–1065.
- Doss, S.; Wandinger, K.P.; Hyman, B.T.; Panzer, J.A.; Synofzik, M.; Dickerson, B.; Mollenhauer, B.; Scherzer, C.R.; Ivinson, A.J.; Finke, C.; et al. High prevalence of NMDA receptor IgA/IgM antibodies in different dementia types. Ann. Clin. Transl. Neurol. 2014, 1, 822–832.
- Smalla, K.H.; Angenstein, F.; Richter, K.; Gundelfinger, E.D.; Staak, A. Identification of fucose alpha(1-2) galactose epitope-containing glycoproteins from rat hippocampus. Neuroreport 1998, 9, 813–817.
- Galili, U.; Buehler, J.; Shohet, S.B.; Macher, B.A. The human natural anti-Gal IgG. III. The subtlety of immune tolerance in man as demonstrated by crossreactivity between natural anti-Gal and anti-B antibodies. J. Exp. Med. 1987, 165, 693–704.
- Pul, R.; Nguyen, D.; Schmitz, U.; Marx, P.; Stangel, M. Comparison of intravenous immunoglobulin preparations on microglial function in vitro: More potent immunomodulatory capacity of an IgM/IgA-enriched preparation. Clin. Neuropharmacol. 2002, 25, 254–259.
- Bredesen, D.E.; Amos, E.C.; Canick, J.; Ackerley, M.; Raji, C.; Fiala, M.; Ahdidan, J. Reversal of cognitive decline in Alzheimer’s disease. Aging 2016, 8, 1250–1258.
- Vossenkämper, A.; Blair, P.A.; Safinia, N.; Fraser, L.D.; Das, L.; Sanders, T.J.; Stagg, A.J.; Sanderson, J.D.; Taylor, K.; Chang, F.; et al. A role for gut-associated lymphoid tissue in shaping the human B cell repertoire. J. Exp. Med. 2013, 210, 1665–1674.
