Monochromatic X-ray has a single energy level in contrast to white X-rays used in conventional radiation therapy. Irradiation of high Z elements such as gadolinium, gold and silver with a monochromatic X-ray can result in photoelectric effects that includes the release of the Auger electrons that have strong cell killing effect. To apply this principle to cancer therapy, various nanoparticles loaded with high Z elements have been developed that enabled high Z elements to be delivered to tumor. The recent addition is gadolinium-loaded mesoporous silica nanoparticle (Gd-MSN). Tumor spheroids have been used as a convenient tumor model to demonstrate that monochromatic X-rays with energy level at or higher than the K-edge energy of gadolinium can destruct tumor mass that has Gd-MSN distributed throughout tumor spheroids.
- monochromatic X-ray
- mesoporous silica nanoparticles
- high-Z elements
- tumor spheroids
1. Definition
While conventional radiation therapy uses white X-rays that consist of a mixture of X-ray waves with various energy levels, a monochromatic X-ray (monoenergetic X-ray) has a single energy level. Irradiation of high-Z elements such as gold, silver or gadolinium with a synchrotron-generated monochromatic X-rays with the energy at or higher than their K-edge energy causes a photoelectric effect that includes release of the Auger electrons that induce DNA damage—leading to cell killing.
2. Introduction
The nanotechnology that was initiated in 1960s has generated a variety of nanomaterials valuable for biomedical applications such as cancer therapy [1]. Of particular interest are nanoparticles—small particles of the size ranging from 40 to 400 nm [2][3][4]. Various materials have been used to generate nanoparticles including organic nanoparticles such as liposomes, synthetic polymers, micelles, protein and biomolecules as well as inorganic nanoparticles such as mesoporous silica nanoparticles, gold nanoparticles and diamond nanoparticles [2][3][4]. Many nanoparticles are efficiently taken up into cancer cells by endocytosis and can accumulate in late endosomes and lysosomes that are localized at the perinuclear region of a cell [5]. In addition, nanoparticles can accumulate in the tumor either via passive enhanced permeability or retention (EPR) mechanism as well as by active targeting mechanisms [2][6].
Convergence of the study on nanotechnology and the study on radiation therapy has resulted in various new approaches to enhance radiation therapy [7][8]. Radiosensitizers have been delivered to the tumor by using nanoparticles. In addition, nanoparticles containing high-Z elements have been developed. Advantageous properties of nanoparticles such as tumor targeting have important implication for radiation therapy. These nanoparticles have been evaluated in tissue culture models, in animal models and in clinical trials. A new addition to these nanoparticles is a type of mesoporous silica nanoparticles that are surface attached with gadolinium [9].
2. Monochromatic X-ray and the Auger Effect
X-rays are electromagnetic waves in the wave length range of one picometer to ten nanometers [10]. Current radiation therapy uses white X-rays which are mixtures of these X-ray waves. By using a monochromator, the white X-ray can be separated into monochromatic X-rays each having a single energy level [11][12] and they can be used for radiation therapy. In addition, monochromatic X-rays have been used for imaging studies [13] to obtain sharper images. Currently, the best source for monochromatic X-ray is to use synchrotrons that generate intense X-ray beams. A synchrotron accelerates electrons to extremely high energy and then direct them to change directions by the use of magnets (bending magnets). The X-ray beams emitted from a bending magnet are directed to beamlines that are placed surrounding the synchrotron ring.
Irradiation of high-Z element such as gold, silver or gadolinium with a monochromatic X-ray causes photoelectric effect involving inner shell ionization. Destabilization of an atom is corrected by the release of photons and electrons.
Figure 1 depicts one outcome that involves the release of the Auger electrons reported by Pierre Auger [10]. In this scenario, when gadolinium is irradiated with the monochromatic X-ray having an energy at or higher than the K-edge energy of gadolinium, an electron in the K-shell will be kicked out of the atom. This results in the movement of an electron from outer shell to the K-shell. This releases energy which is then used to kick out other electrons from the atom. Thus, the series of events lead to the release of electrons. If an element other than gadolinium is used, then the monochromatic X-ray energy needs to be adjusted so that it is higher than the K-edge energy of the particular element. The electrons released are called the Auger electrons that possess strong cell killing effect that involves DNA strand breaks by direct effect as well as by indirect effect mediated by radicals. In addition, cell membrane damages may be induced by the Auger electrons. In addition, a bystander effect on non-exposed cells could occur. However, due to short mean free path, the effect is relatively confined within the cancer cell. Occurrence of these electrons was reported in 1922 by Lise Meitner (discussed in [18]).depicts one outcome that involves the release of the Auger electrons reported by Pierre Auger [14]. In this scenario, when gadolinium is irradiated with the monochromatic X-ray having an energy at or higher than the K-edge energy of gadolinium, an electron in the K-shell will be kicked out of the atom. This results in the movement of an electron from outer shell to the K-shell. This releases energy which is then used to kick out other electrons from the atom. Thus, the series of events lead to the release of electrons. If an element other than gadolinium is used, then the monochromatic X-ray energy needs to be adjusted so that it is higher than the K-edge energy of the particular element. The electrons released are called the Auger electrons that possess strong cell killing effect that involves DNA strand breaks by direct effect as well as by indirect effect mediated by radicals. In addition, cell membrane damages may be induced by the Auger electrons. In addition, a bystander effect on non-exposed cells could occur. However, due to short mean free path, the effect is relatively confined within the cancer cell. Occurrence of these electrons was reported in 1922 by Lise Meitner (discussed in [15]).
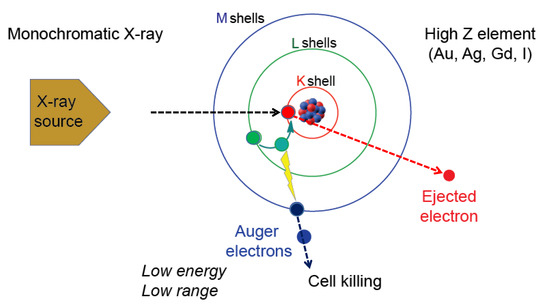
The Auger effect. Irradiation of high-Z element such as Au, Ag, Gd or I with a monochromatic X-ray having a defined energy level causes effects that include ejection of a K-shell electron (red circle). One of the ways to reestablish energy equilibrium is the movement of an electron from a higher shell (green circle) causing the release of energy that is used by another electron (blue circle) which is released as an Auger electron. The Auger electron has strong cell killing effect.
Thus, the effect of monochromatic X-rays can be amplified by the use of high-Z elements, raising the possibility that the Auger effect-based cancer therapy can be developed. There has been a quest to develop the Auger therapy (reviewed in [15][16][17][18][19][20]). In early studies, biologic effect of
125I-nucleotides incorporated into DNA was examined, as they undergo natural decay resulting in the release of Auger electrons. The study uncovered cell killing effect including DNA cleavage. Subsequently, photon-activation therapy (PAT) was examined. Various studies using cancer cells as well as animal tumor models have been carried out [18,19,20,21].I-nucleotides incorporated into DNA was examined, as they undergo natural decay resulting in the release of Auger electrons. The study uncovered cell killing effect including DNA cleavage. Subsequently, photon-activation therapy (PAT) was examined. Various studies using cancer cells as well as animal tumor models have been carried out [15][16][17][18].
4. Summary
Monochromatic X-rays provide a valuable source for radiation therapy. This special type of X-ray with a single energy level can be obtained by separating a synchrotron-generated white X-ray by the use of a monochromator. Irradiation of high-Z elements such as gadolinium causes photoelectric effect including the release of Auger electrons. Various nanoparticles containing gadolinium have been developed for their effect to enhance radiation therapy and a recent addition to the growing list of such nanoparticles is gadolinium-loaded mesoporous silica nanoparticles (Gd–MSNs). These nanoparticles differ in their size and composition. Additional nanoparticles may be developed in the future. Thus, it will be important to evaluate their ability to enhance effect of X-ray irradiation. While cancer cell models and animal models will continue to be important, tumor organoid models provide versatile and convenient assays to examine their ability to cause destruction upon X-ray irradiation.Monochromatic X-rays provide a valuable source for radiation therapy. This special type of X-ray with a single energy level can be obtained by separating a synchrotron-generated white X-ray by the use of a monochromator. Irradiation of high-Z elements such as gadolinium causes photoelectric effect including the release of Auger electrons. Various nanoparticles containing gadolinium have been developed for their effect to enhance radiation therapy and a recent addition to the growing list of such nanoparticles is gadolinium-loaded mesoporous silica nanoparticles (Gd–MSNs). These nanoparticles differ in their size and composition. Additional nanoparticles may be developed in the future. Thus, it will be important to evaluate their ability to enhance effect of X-ray irradiation. While cancer cell models and animal models will continue to be important, tumor organoid models provide versatile and convenient assays to examine their ability to cause destruction upon X-ray irradiation.
References
- Maurya, A.; Singh, A.K.; Mishra, G.; Kumari, K.; Rai, A.; Sharma, B.; Kulkarni, G.T.; Awasthi, R. Strategic use of nanotechnology in drug targeting and its consequences on human health: A focused review. Interv. Med. Appl. Sci. 2019, 11, 38–54.
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79.
- Naz, S.; Shamoon, M.; Wang, R.; Zhang, L.; Zhou, J.; Chen, J. Advances in therapeutic implications of inorganic drug delivery Nano-Platforms for Cancer. Int. J. Mol. Sci. 2019, 20, 965.
- Vallet-Regi, M.; Tamanoi, F. Chapter One-Overview of studies regarding mesoporous silica nanomaterials and their biomedical application. In The Enzymes; Academic Press: London, UK, 2018; Volume 43, pp. 1–10.
- Patel, S.; Kim, J.; Herrera, M.; Mukherjee, A.; Kabanov, A.V.; Sahay, G. Brief update on endocytosis of nanomedicines. Adv. Drug Deliv. Rev. 2019, 144, 90–111.
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284.
- Boateng, F.; Ngwa, W. Delivery of nanoparticle-based radiosensitizers for radiotherapy applications. Int. J. Mol. Sci. 2019, 21, 273.
- K. Bakht, M.; Sadeghi, M.; Pourbaghi-Masouleh, M.; Tenreiro, C. Scope of nanotechnology-based radiation therapy and thermotherapy methods in cancer treatment. Curr. Cancer Drug Targets 2012, 12, 998–1015.
- Matsumoto, K.; Saitoh, H.; Doan, T.L.H.; Shiro, A.; Nakai, K.; Komatsu, A.; Tsujimoto, M.; Yasuda, R.; Kawachi, T.; Tajima, T.; et al. Destruction of tumor mass by gadolinium-loaded nanoparticles irradiated with monochromatic X-rays: Implications for the Auger therapy. Sci. Rep. 2019, 9, 1–10.
- Bushberg, J.T.; Seibert, J.A.; Leidholdt Jr, E.M.; Boone, J.M. The essential physics of medical imaging; Williams & Wilkins: Philadelphia, PA, US, 1994.
- Lewis, R. Medical applications of synchrotron radiation x-rays. Phys. Med. Biol. 1997, 42, 1213–1243.
- Burattini, E.; Gambaccini, M.; Indovina, P.L.; Pocek, M.; Simonetti, G. Synchrotron radiation: A new source in x-ray mammography. Radiol. Med. 1992, 84, 181–188.
- Pole, D.; Popovic, K.; Williams, M. Contrast imaging with a monochromatic x-ray scanner. In Proceedings of the Medical Imaging, San Diego, CA, USA, 18 March 2008. Lawaczeck, R.; Arkadiev, V.; Diekmann, F.; Krumrey, M. Monochromatic x-rays in digital mammography. Invest. Radiol. 2005, 40, 33–39.
- Auger, P. The Auger effect. Surf. Sci. 1975, 48, 1–8.
- Martin, R.F.; Feinendegen, L.E. The quest to exploit the Auger effect in cancer radiotherapy—A reflective review. Int. J. Radiat. Biol. 2016, 92, 617–632.
- Yokoya, A.; Ito, T. Photon-induced Auger effect in biological systems: A review. Int. J. Radiat. Biol. 2017, 93, 743–756.
- Fairchild, R.G.; Brill, A.B.; Ettinger, K.V. Radiation enhancement with iodinated deoxyuridine. Invest. Radiol. 1982, 17, 407–416.
- Biston, M.C.; Joubert, A.; Adam, J.F.; Elleaume, H.; Bohic, S.; Charvet, A.M.; Esteve, F.; Foray, N.; Balosso, J. Cure of Fisher rats bearing radioresistant F98 glioma treated with cis-platinum and irradiated with monochromatic synchrotron X-rays. Cancer Res. 2004, 64, 2317–2323.
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger electrons for cancer therapy-a review. EJNMMI Radiopharm Chem. 2019, 4, 1–36.
- Kassis, A.I.; Adelstein, S.J. Radiobiologic principles in radionuclide therapy. J. Nucl. Med. 2005, 46 (Suppl. 1), 4S–12S.
