Retinal vein occlusion (RVO) is the second most common retinal vascular disorder next to diabetic retinopathy.
- retinal vein occlusion
- macular edema
- microaneurysm
1. Introduction
Retinal vein occlusion (RVO) is the second most common retinal vascular disorder next to diabetic retinopathy. In most patients with RVO, macular edema (ME) is the pre-dominant cause of visual loss in the acute and chronic stages. Intravitreal (IV) injection of anti-vascular endothelial growth factor (VEGF) agents has revolutionized the therapy for ME associated with RVO [1][2]. However, the frequent recurrence of ME after anti-VEGF therapies highlight the need for better understanding of the pathology of ME and development of a therapy based on the pathology. Various microvascular abnormalities (Figure 1) are observed in eyes with RVO, which complicate the pathology in eyes with RVO.
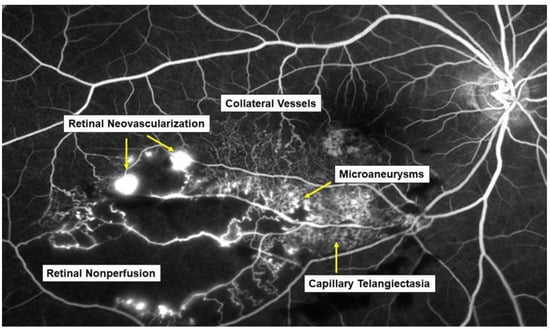
Figure 1. Various microvascular abnormalities associated with branch retinal vein occlusion (BRVO) on a fluorescein angiogram. In the chronic stage, various microvascular abnormalities are observed in an eye with BRVO.
2. Mechanism of Retinal Vein Occlusion
2.1. Pathogenesis of Retinal Vein Occlusion
The pathogenesis of RVO is multifactorial. Arteriosclerosis strongly contributes to the onset of RVO. Arteriosclerosis results in vein occlusion through endothelial cell damage and thrombosis. For instance, a rigid artery compresses the underlying venous wall at the arteriovenous (AV) crossings, resulting in disturbance of venous return and changes in the course of venous flow, turbulent blood flow, chronic damage to the retinal vascular endothelial cells, and thrombosis formation, which result in onset of RVO. A thrombosis is thought to be caused by the three important factors of Virchow’s triad, i.e., endothelial injury, hypercoagulability, and abnormal blood flow (Figure 2). It is reasonable that these three factors strongly contribute to the pathology of RVO.
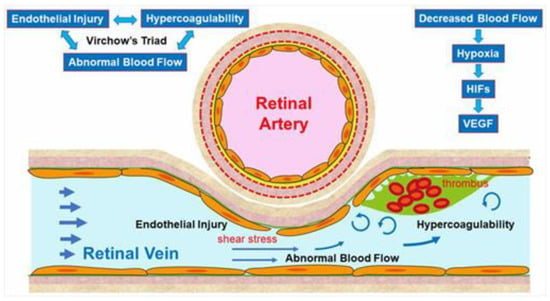
Figure 2. Mechanism of retinal vein occlusion (RVO). A rigid artery compresses the underlying venous wall at the arteriovenous crossings, resulting in disturbing the venous return and changing the course of the venous flow, turbulent blood flow, causing chronic damage to the retinal vascular endothelial cells, and thrombosis formation, which result in the onset of RVO. A thrombosis is thought to be caused by the three important factors of Virchow’s triad, i.e., endothelial injury, hypercoagulability, and abnormal blood flow. HIF: hypoxia inducible factor, VEGF: vascular endothelial growth factor.
Increased vascular pressure behind the occlusion may lead to leakage of fluid across the vascular wall to the adjacent retinal tissue [3][4][5][6]. Furthermore, the damage of the endothelium in the affected vein may induce a low-grade, chronic inflammation of the retinal microvasculature and an upregulation of inflammatory mediators that break the blood–retina barrier and perpetuate ME [3][4][5][6].
Another hypothesis is that arteriosclerosis results in arterial insufficiency, leading to RVO. Arterial insufficiency, i.e., insufficient oxygen transport to the retina due to arteriosclerosis, can cause retinal hypoxia, resulting in production of VEGF, which plays a central role in the onset of and various microvascular abnormalities in RVO.
Multiple studies have established a significance of AV crossings in eyes with branch retinal vein occlusion (BRVO) [7][8][9]. Namely, the artery and vein share the common adventitial sheath at the AV crossings and the rigid artery causes a mechanical obstruction of the vein (Figure 2). Further, thickened vessel walls and narrowed venous lumina also result in RVO, as a histological study reported that used postmortem tissue from a patient with central RVO (CRVO) [10]. Previous studies using OCT also have shown that the retinal veins narrowed at the AV crossings [11]. The paper described that in all eyes with BRVO of the vein overcrossing, the vein appeared to be compressed and choked between the internal limiting membrane and the arterial wall at the AV crossing, whereas the venous lumen was generally preserved at the AV crossing in eyes with arterial overcrossing [11], suggesting that other factors such as shear stress due to abnormal blood flow and/or endothelial injury might cause the pathogenesis of BRVO (Figure 2).
2.2. Endothelial Injury and Thickening of the Vessel Walls
Vascular endothelial cells maintain smooth microcirculation and play a central role in abnormal microcirculation (Figure 2). Vascular endothelial cells usually regulate vascular resistance, i.e., prevent thrombosis formation by producing vasodilator and vasoconstrictor mediators such as nitric oxide (NO), prostaglandin I2 (PGI2), and endothelin. However, chronic stress to the endothelium due to hypertension, diabetes mellitus, and hyperlipidemia causes arteriosclerosis. A mechanical obstruction of the vein at the AV crossings results in venous blood stasis, turbulent blood flow, endothelial injury, thickening of the intima media, and formation of a vascular thrombosis. A histologic study using postmortem tissue from a patient with CRVO [10] reported thickened vessel walls and narrowed venous lumens. Furthermore, previous studies using OCT have also shown that the venous lumen narrowed at the AV crossings in eyes with BRVO [11]. Loss of capillary endothelial cells and pericytes has been reported in previous histological studies [10][12]. Moreover, retinal vascular endothelial cells form a tight junction with each other, function as an inner blood–retina barrier, and maintain the retinal stability. Therefore, the retinal vascular endothelial injury and consequent blood–retina barrier breakdown cause vascular hyperpermeability and the retina becomes edematous. VEGF also has been reported to increase vessel permeability by increasing the phosphorylation of tight junction proteins and was thus an important mediator of the blood–retina barrier breakdown leading to vascular leakage and ME [13]. We can indirectly evaluate the endothelial injury using FA, which can image the hyperpermeability (Figure 3).
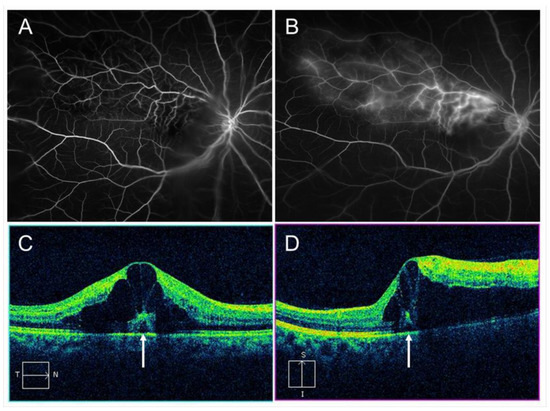
Figure 3. Macular edema (ME) associated with branch retinal vein occlusion. (A) Early phase of fluorescein angiogram. (B) Late phase of fluorescein angiogram. The hyperfluorescence on (B) indicates pooling due to hyperpermeability from retinal vessels. (C) Horizontal scan of optical coherence tomography (OCT) image. (D) Vertical scan of OCT image. Cystoid macular edema and tiny serous retinal detachment (white arrows) are observed.
2.3. Hypercoagulability
Vascular endothelial cells control platelet function and blood coagulation and fibrinolysis. NO and PGI2 are produced by endothelial cells and not only cause vasodilation but also impede platelet aggregation and control the microvascular circulation by interacting with each other. Therefore, venous blood stasis at the AV crossings damages the retinal vascular endothelial cells, leading to decreased production of NO and PGI2 and increased production of tissue factors, resulting in hypercoagulability and acceleration of thrombosis formation (Figure 2).
2.4. Abnormal Blood Flow
Yoshida et al. [14], who measured retinal blood vessel diameters, retinal blood flow, and the absolute retinal blood velocity of the 18 arterial sites and 18 venous sites between the optic disc margin and the first bifurcation in healthy subjects and a patient with RVO using a laser Doppler flowmeter, reported that the blood velocities in the eye with BRVO and the fellow eye decreased compared with that in a normal eye. Moreover, the flow characteristics in a patient with CRVO improved dramatically after the occlusion resolved. Yamada et al. [15] also reported that the mean blur rate of the major vessels around the optic disc in eyes with CRVO measured by laser speckle flowgraphy decreased compared with that in the fellow eye and was correlated inversely with the aqueous VEGF concentration level. The oxygen saturation in first- and second-degree retinal venules also decreased in the patient with CRVO [16]. Since the bloodstream transports both nourishment and oxygen, decreased retinal blood flow can cause hypoxia in the retina, leading to production of hypoxia-inducible factors and consequent VEGF expression (Figure 2).
Blood flow also is associated with shear stress on the vessel walls, i.e., appropriate shear stress on the vessel walls causes endothelial cells to secret NO, which prevents thrombosis formation by controlling platelet aggregation and leukocyte adhesion to the vessel walls. In contrast, decreased shear stress on the vessel walls results in decreased inflammatory cytokines and cell adhesion molecules and the increased possibility of leukocyte adhesion and thrombosis formation (Figure 2).
References
- Campochiaro, P.A.; Heier, J.S.; Feiner, L.; Gray, S.; Saroj, N.; Rundle, A.C.; Murahashi, W.Y.; Rubio, R.G.; BRAVO Investigators. Ranibizumab for macular edema following branch retinal vein occlusion: Six-month primary end points results of a phase III study. Ophthalmology 2010, 117, 1102–1112.
- Brown, D.M.; Campochiaro, P.A.; Bhisitkul, R.B.; Ho, A.C.; Gray, S.; Saroj, N.; Adamis, A.P.; Rubio, R.G.; Murahashi, W.Y. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology 2011, 118, 1594–1602.
- Funk, M.; Kriechbaum, K.; Prager, F.; Benesch, T.; Georgopoulos, M.; Zlabinger, G.J.; Schmidt-Erfurth, U. Intraocular concentra- tions of growth factors and cytokines in retinal vein occlusion and the effect of therapy with bevacizumab. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1025–1032.
- Corvi, F.; Spina, C.L.; Benatti, L.; Querques, L.; Lattanzio, R.; Bandello, F.; Querques, G. Impact of intravitreal ranibizumab on vessel functionality in patients with retinal vein occlusion. Am. J. Ophthalmol. 2015, 160, 45–52.
- Corvi, F.; Querques, G.; Spina, C.L.; Lattanzio, R.; Bandello, F. Dynamic and static retinal vessel analyses in patients with macular edema secondary to retinal vein occlusion. Retina 2015, 35, 2052–2059.
- Eibenberger, K.; Schmetterer, L.; Rezar-Dreindl, S.; Wozniak, P.; Told, R.; Mylonas, G.; Krall, C.; Schmidt-Erfurth, U.; Sacu, S. Effects of intravitreal dexamethasone implants on retinal oxygen saturation, vessel diameter, and retrobulbar blood flow velocity in ME secondary to RVO. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5022–5029.
- Duker, J.S.; Brown, G.C. Anterior location of the crossing artery in branch retinal vein obstruction. Arch Ophthalmol. 1989, 107, 998–1000.
- Weinberg, D.; Dodwell, D.G.; Fern, S.A. Anatomy of arteriovenous crossings in branch retinal vein occlusion. Am. J. Ophthalmol. 1990, 109, 298–302.
- Christoffersen, N.L.; Larsen, M. Pathophysiology and hemodynamics of branch retinal vein occlusion. Ophthalmology 1999, 106, 2054–2062.
- Powner, M.B.; Sim, D.A.; Zhu, M.; Nobre-Cardoso, J.; Jones, R.; Syed, A.; Chang, A.A.; Keane, P.A.; Tufail, A.; Egan, C.A.; Fruttiger, M. Evaluation of Nonperfused Retinal Vessels in Ischemic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5031–5037.
- Muraoka, Y.; Tsujikawa, A.; Murakami, T.; Ogino, K.; Kumagai, K.; Miyamoto, K.; Uji, A.; Yoshimura, N. Morphologic and functional changes in retinal vessels associated with branch retinal vein occlusion. Ophthalmology 2013, 120, 91–99.
- Frangieh, G.T.; Green, W.R.; Barraquer-Somers, E.; Finkelstein, D. Histopathologic study of nine branch retinal vein occlu-sions. Arch Ophthalmol. 1982, 100, 1132–1140.
- Noma, H.; Funatsu, H.; Yamasaki, M.; Tsukamoto, H.; Mimura, T.; Sone, T.; Jian, K.; Sakamoto, I.; Nakano, K.; Yamashita, H.; et al. Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular en- dothelial growth factor and interleukin-6. Am. J. Ophthalmol. 2005, 140, 256–261.
- Yoshida, A.; Feke, G.T.; Mori, F.; Nagaoka, T.; Fujio, N.; Ogasawara, H.; Konno, S.; Mcmeel, J.W. Reproducibility and clinical application of a newly developed stabilized retinal laser Doppler instrument. Am. J. Ophthalmol. 2003, 135, 356–361.
- Yamada, Y.; Suzuma, K.; Matsumoto, M.; Tsuiki, E.; Fujikawa, A.; Harada, T.; Kitaoka, T. Retinal blood flow correlates to aqueous vascular endothelial growth factor in central retinal vein occlusion. Retina 2015, 35, 2037–2042.
- Hardarson, S.H.; Stefánsson, E. Oxygen saturation in central retinal vein occlusion. Am. J. Ophthalmol. 2010, 150, 871–875.
