Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Felisa Reyes-Ortega and Version 3 by Conner Chen.
Apart from the more common ocular disorders, there are some genetic diseases, such as cystic fibrosis, that develop ocular disorders as secondary effects as long as the disease progresses. In those cases, Magnetic Nanoparticles can be used as potent drug carriers and magnetic hyperthermia agents due to their response to an external magnetic field.
- ocular disorders
- ocular cancer
- cystic fibrosis
- magnetic nanoparticles
- therapeutic agents
1. Ocular Barriers for Drug Delivery Systems
Initially, the anatomical barrier that a drug finds in the eye is the cornea (Figure 1). Cornea thickness is from 551 to 565 µm in the center section and from 612 to 640 µm in the periphery, and is formed by five layers: epithelium, Bowman’s layer, stroma, Descemet membrane, and the endothelium [1]. The cornea contains barriers to both hydrophilic and hydrophobic molecules. Corneal epithelium is the first anatomical barrier, which consists of a basal layer of columnar cells surrounded by intercellular tight junctions [2]. Corneal epithelium is negatively charged at physiological pH and the tight junctions act as barriers for the permeation of hydrophilic drugs, such as fluoroquinolones (e.g., norfloxacin) or intraocular pressure (IOP) beta-blockers (e.g., timolol). After the epithelium, the second cornea layer that can be an impediment for drug diffusion is the stroma (Figure 1), which is formed by multiple layers of hexagonally arranged collagen fibers containing aqueous pores that allow hydrophilic drugs to easily pass through but acting as a significant barrier for lipophilic drugs [3], such as prostaglandin (e.g., latanaprost) or NSAIDs (e.g., celecoxib). Consequently, drug bioavailability depends of the balance between lipophilicity and hydrophilicity to avoid being retained in the corneal barriers. Recently, it has been observed that the use of MNPs coated with chitosan can enhance the corneal residence time and drug contact [4], since the chitosan coating acts as a permeability enhancer.
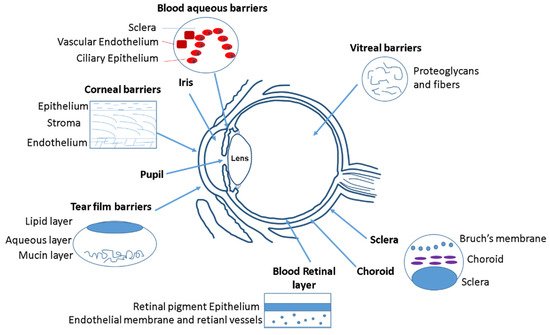
Figure 1. Different ocular barriers for drug delivery systems.
After the cornea, the next anatomical barrier is the iris and ciliary body (Figure 1). The iris is composed of a pigmented stromal layer formed by epithelial cells. The ciliary body is formed by ciliary muscles and ciliary epithelial cells that produce aqueous humor, the transparent protein-containing fluid that nourishes the cornea and lens [5]. The constant secretion, flow, and drainage of aqueous humor controls the IOP of the eye. Then, MNPs that are able to penetrate the cornea need to diffuse against the flow of the aqueous humor and face elimination via Schlemm’s canal [5]. Another anatomical barrier is the lens that is formed by four structures: lens capsule, epithelium, cortex and nucleus. The epithelial cells that formed the capsule generates a barrier to hydrophilic molecules. The cortex and nucleus are made by lens fibers, whose tightly compact arrangement limit drug diffusion in the lens [6]. Conjunctiva and sclera are other anatomical barriers of the eyes, whose composition is basically collagenous and elastic fibers, limiting, such as in the cornea, the drug diffusion [7] (Figure 1). The conjunctiva runs 3–5 cell layers thick, with tight junctions on the apical surface. The sclera is a fibrous, opaque tissue forming the outer layer of the eye and is continuous with the cornea. Choroid is a layer of vasculature lying between the retina and sclera and it contains Bruch’s membrane, which is formed by several layers of collagenous and elastic fibers and forms the basement membrane for retinal pigment epithelium. Due to the systemic circulation of this layer, there is a rapid clearance of the drug delivery systems [8].
The retina is anatomically the back layer of the eye and is composed of the blood–retinal barrier (BRB) (Figure 1). The inner part of the BRB is formed by tight junctions between the retinal capillary endothelial cells, and the outer-blood–retinal barrier separates the choroid and Bruch’s membrane from the inner retina. Drug delivery systems must be able to cross the inner and outer membranes of the retina, which pose a strict physical barrier for most polymeric nanoparticles. Magnetic nanoparticles can enhance the retina layers penetration due to their magnetic response under the application of an external magnetic field [9][10].
The main physiological barriers of the eyes are its tear film and nasolacrimal drainage. The lacrimal fluid is an isotonic aqueous solution that contains a mixture of proteins and lipids. The lacrimation and nasolacrimal drainage decrease the drug exposure time during their administration, and they are responsible for a significant loss of topically applied drugs [11]. Another physiological barrier is related to the efflux proteins, which are located on the apical or basolateral cell membranes of the corneal epithelium, in conjunctiva, and in the iris ciliary body [12]. These proteins can also affect to the absorption of different drugs. Mainly there are two efflux pumps responsible for drug resistance: the P-glycoprotein that limit the entry of amphipathic drugs, and the multidrug-resistant protein (MRP), which acts as an organic anionic transporter [13].
2. Ocular Delivery Routes and Their Limitations
Focusing in the biodistribution of the magnetic nanoparticles (MNPs) administrated to the eye, several factors have to be taken into account during MNPs synthesis, such as the magnetic surface properties, size, suspension media, and administration route. Different drug delivery routes can be used to inject nanoparticles into the eyes, such as topical, systemic, punctal, subconjunctival, intrascleral, fornix, sub-Tenon’s, suprachoroidal, subretinal, and intravitreal injection [14] (Figure 2).
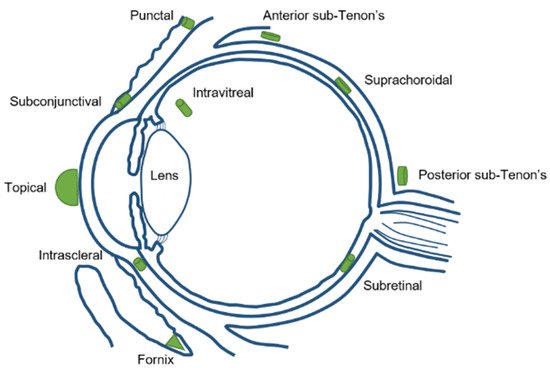
Figure 2. Different ocular drug delivery routes.
Topical application has the limitation of reaching a high ocular bioavailability as a large portion of the compound applied would be lost due to dilution of tears and lacrimation. Usually, the amount of drug that reaches the aqueous humor is less than 5% (Figure 3). Sustained release of the drug is difficult and forces one to markedly increase the drug dose administrated in order to get an optimum therapeutic efficacy.
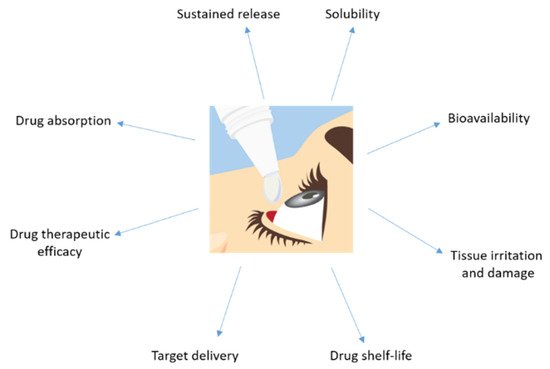
Figure 3. Major disadvantages showed by a drug topically administrated to the eye.
Systemic injection also has the same limitation of a low amount of drug available in the specific site (the eye), especially if the drug is more hydrophilic. It would require more frequent systemic injections to reach and maintain a therapeutic dose, which creates more side effects in the body. The periocular injection can refer to posterior juxtascleral, subconjunctival, retrobulbar, peribulbar, or subtenon injection. Some risks associated for periocular injections are hyphemia, an increase in intraocular pressure, corneal decompensation, and even strabismus. Intravitreal injections are becoming a more popular choice for ocular drug delivery. By micro-needle injection of the compound directly into the vitreous, intravitreal injection could offer a higher drug load in the retina and vitreous compared to other delivery methods. Nowadays, there are some intravitreal products in the market, such as Avastin® (Genentech, San Francisco, CA, USA), Lucentis® (Genentech, San Francisco, CA, USA), Macugen® (Eyetech Inc., Boca Raton FL, USA), and Triesence®(Alcon, Vernier-Geneva, Switzerland) [15]. The drug’s molecular weight is a major factor affecting drug elimination for intravitreal injection. The disadvantages of intravitreal injection include development of certain complications, such as intravitreal hemorrhages, endophthalmitis, and retinal detachment. Patients with diseases affecting the posterior segment usually need multiple intravitreal injections and follow careful monitoring [16].
3. Ocular Disorders Derived from Cystic Fibrosis Disease
The corneal epithelium contributes to fluid transport via various ion channels, from the stroma to the pre-corneal tear film. Numerous channels in the corneal epithelium are responsible of the fluid transport in the eye (Figure 4). Located on the basolateral membrane, the sodium:potassium:chloride (Na+:K+:2Cl−) co-transporter functions in parallel with the sodium:potassium (Na+:K+) pump resulting in CF influx. The rate of secretion by these channels is regulated by the CF conductance of the apical membrane. In addition to CFTR, calcium-activated CF channels (CLCA2) are also thought to contribute to CF efflux. At the ocular surface, basal CFTR activity is likely minimal, as CF patients with loss-of-function CFTR mutations suffer only from mild tear film abnormalities [17][18][19][20]. However, the CFTR-facilitated Cl− transport at the ocular surface provides a rational basis for the investigation of CFTR modulators, e.g., ivacaftor. Currently, eye manifestations of CF are less well known; however, mounting evidence suggests that ocular disorders in CF are a serious problem. A number of ocular disorders derived from CF have been reported, including xerophthalmia, papilledema, and retinal hemorrhages [21]. Joshi et al. [22] reported the first case of newly diagnosed CF-related liver disease in a teenage boy presenting symptoms of night blindness secondary to vitamin A deficiency. In CF, the malabsorption of fat-soluble vitamins, e.g., vitamin A, reduces the concentration of the retinol-binding protein that is essential for the liver-to-tissue transport of retinol. Night blindness is the first sign of vitamin A deficiency with further symptoms after prolonged periods of deficiency, such as Bitot’s spots; triangular, perilimbal grey plaques of keratinized conjunctival debris; and xerosis and dry granular patches. Furthermore, patients with CF have been reported to develop retinal vein occlusions [23][24][25]. It has been hypothesized that elevated fibrinogen levels due to chronic infections or increased homocysteine levels predispose patients with CF to develop retinal vein occlusions.
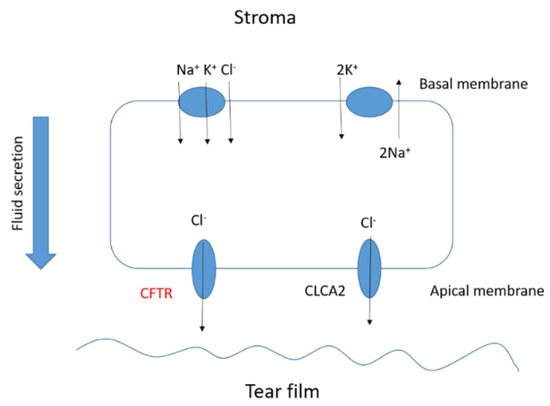
Figure 4.
Basic scheme of the fluid transport in a corneal epithelial cell.
Other ocular disorders related to CF disease are differences in the morphology of the cornea, a reduction in the endothelial cell area, an increase in corneal thickness, an increase in the endothelial cell density and permeability, and an increase in the endothelial pump rate. Lass et al. [26] observed morphological differences in the corneal endothelium in CF patients and CF-related diabetes patients. Mean corneal thickness was significantly greater for both CF groups compared to a safe patient and, therefore, the corneal endothelial permeability and mean relative pump rate were significantly higher in the two CF groups. The increased corneal thickness observed in the CF group suggests there is only partial compensation by the increased pump rate for the increased permeability. Several studies have investigated the incidence of blepharitis in patients with CF [27]. Mrugacz et al. [28] suggested increased blepharitis could indicate lipid dysfunction in CF and that meibomian dysfunction was consistent with the glandular dysfunction observed in CF. Conjunctival xerosis is characterized by keratinization and drying of the conjunctiva due to loss of goblet cells and basal cell proliferation. This incidence of conjunctival xerosis in CF has been reported by several authors [29][30][31]. Macular pigment is derived from two carotenoids, lutein and zeaxanthin. Both the serum lutein and zeaxanthin concentrations as well as the macular pigment optical density were observed to be significantly lower in CF patients [32]. Even at a very early stage of CF disease development (newborns), ocular disorders have been observed [33][34]. As babies with CF frequently present with lower birth weights (which itself has been associated as a risk factor for ametropia, strabismus, and amblyopia), early and regular eye examinations for all children with CF remain essential.
4. MNPs as Carriers for Drug Delivery
Magnetic nanoparticles differ from the rest of the nanocarriers due to their magnetic properties that make them unique for drug delivery. Drugs molecules can be conjugated to the shell of magnetic nanoparticles to be injected into the body and be concentrated in a local area (avoiding the damage to other tissues) due to the effect of an external magnetic field. Owing to the MNPs large surface-to-volume ratio, it offers numerous chemically active sites for biomolecule conjugation [35]. It helps to increase the drug circulation time into the organism and to get the target site. Furthermore, these functionalized magnetic nanoparticles can act as hyperthermia agents, providing a more potent therapeutic effect since the increase in temperature in a specific site promote tumor cell death without altering normal cells [36][37]. Besides, magnetic nanoparticles can be easily visualized by magnetic resonance imaging (useful for diagnosis) by the application of an external magnetic field [38]. The most frequent magnetic nanoparticles used in biomedical applications are magnetite (Fe3O4) or maghemite (ɣFe2O3) due to their higher biocompatibility and stability [39]. The physicochemical properties of MNPs (such as size, shape, charge, and anisotropy) also are known to affect cellular responses, such as the internalization rates and mechanisms or cytotoxicity [40]. It is crucial to predict all these parameters in the nanoparticles synthesis to get the maximum therapeutic treatment. Iron oxide nanoparticles (IONPs) have been described to be easily modulated in terms of size, shape, and magnetic hyperthermia response.
Several research works described the use of MNPs in vitro and in vivo as therapeutic agents for the treatment of certain ocular tumors, such as retinoblastoma, uveal melanoma, choroidal melanoma, and choroidal hemangioma. For example, Demirci et al. demonstrated that IONPs can act as efficient therapeutic nanoheaters able to target exclusively the drug delivery in the eye by intravitreal injection and the application of an external magnetic field for the treatment of retinoblastoma [41]. The IONPs used in this study were coated with dextran and were tested in the Y79 retinoblastoma cell line, resulting in selectively killing retinoblastoma tumor cells via the activation of apoptotic pathways. Latorre et al. used gold nanoclusters coated with albumin for the treatment of uveal melanoma [42]. These nanoclusters were loaded with the AZD8055 drug, a selective inhibitor of mTOR that prevents the proliferation of uveal melanoma tumor cells. The therapeutic efficacy was tested in vitro and compared with non-tumoral keratinocytes, showing the high selectivity of the tumor cells. These AZD8055 nanoculsters were tested in vivo, using a mouse model, demonstrating the potential for stopping uveal melanoma metastasis. Giannaccini et al. demonstrated that intravitreally injected MNPs were able to localize rapidly in the retinal pigment epithelium (RPE) as a potent therapeutic tool for the treatment of choroidal melanoma [43]. These MNPs were functionalized with the vascular endothelial growth factor to produce transcytosis from the RPE towards more posterior layers in the eye. Orynbayeva et al. studied the internalization of polymeric-coated MNPs on primary rat endothelial cells, showing that MNPs are potent targeting and therapeutic agents that did not affect the structural integrity and functionality of the primary endothelial cells [44]. Yanai et al. used IONPS for intravitreal injection or via the tail vein in a transgenic rat model of the retina, showing the efficacy of targeting the upper hemisphere of the rodent retina [45].
References
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194.
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers-Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28.
- Kwatra, D.M.A. Drug delivery in ocular diseases: Barriers and strategies. World J. Pharmacol. 2013, 2, 78–83.
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and its Derivatives for Ocular Delivery Formulations: Recent Advances and Developments. Polymers 2020, 12, 1519.
- Delamere, N.A. Ciliary Body and Ciliary Epithelium. Adv. Organ. Biol. 2005, 10, 127–148.
- Swetledge, S.; Jung, J.P.; Carter, R.; Sabliov, C. Distribution of polymeric nanoparticles in the eye: Implications in ocular disease therapy. J. Nanobiotechnol. 2021, 19, 10.
- Sánchez-López, E.; Espina, M.; Doktorovova, S.; Souto, E.B.; García, M.L. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye—Part I—Barriers and determining factors in ocular delivery. Eur. J. Pharm. Biopharm. 2017, 110, 70–75.
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-Álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269.
- Giannaccini, M.; Giannini, M.; Calatayud, M.P.; Goya, G.F.; Cuschieri, A.; Dente, L.; Raffa, V. Magnetic Nanoparticles as Intraocular Drug Delivery System to Target Retinal Pigmented Epithelium (RPE). Int. J. Mol. Sci. 2014, 15, 1590–1605.
- Giannaccini, M.; Pedicini, L.; De Matienzo, G.; Chiellini, F.; Dente, L.; Raffa, V. Magnetic nanoparticles: A strategy to target the choroidal layer in the posterior segment of the eye. Sci. Rep. 2017, 7, 43092.
- Awwad, S.; Ahmed, A.M.; Sharma, G.; Heng, J.; Khaw, P.T.; Brocchini, S.; Lockwood, A. Principles of pharmacology in the eye. Br. J. Pharmacol. 2017, 174, 4205–4223.
- Zhang, T.; Xiang, C.D.; Gale, D.; Carreiro, S.; Wu, E.Y.; Zhang, E.Y. Drug Transporter and Cytochrome P450 mRNA Expression in Human Ocular Barriers: Implications for Ocular Drug Disposition. Drug Metab. Dispos. 2008, 36, 1300–1307.
- Aukunuru, J.; Sunkara, G.; Bandi, N.; Thoreson, W.; Kompella, U.B. Expression of Multidrug Resistance-Associated Protein (MRP) in Human Retinal Pigment Epithelial Cells and Its Interaction with BAPSG, a Novel Aldose Reductase Inhibitor. Pharm. Res. 2001, 18, 565–572.
- Joseph, R.R.; Venkatraman, S.S. Drug delivery to the eye: What benefits do nanocarriers offer? Nanomedicine 2017, 12, 683–702.
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754.
- You, S.; Luo, J.; Grossniklaus, H.E.; Gou, M.-L.; Meng, K.; Zhang, Q. Nanomedicine in the application of uveal melanoma. Int. J. Ophthalmol. 2016, 9, 1215–1225.
- Botelho, S.Y.; Goldstein, A.M.; Rosenlund, M.L. Tear sodium, potassium, chloride, and calcium at various flow rates: Children with cystic fibrosis and unaffected siblings with and without corneal staining. J. Pediatr. 1973, 83, 601–606.
- Morkeberg, J.C.; Edmund, C.; Prause, J.U.; Lanng, S.; Koch, C.; Michaelsen, K.F. Ocular findings in cystic fibrosis patients receiving vitamin A supplementation. Graefe’s Arch. Clin. Exp. Ophthalmol. 1995, 233, 709–713.
- Mrugacz, M.; Kaczmarski, M.; Bakunowicz-Lazarczyk, A.; Zelazowska, B.; Wysocka, J.; Minarowska, A. IL-8 and IFN-γ in Tear Fluid of Patients with Cystic Fibrosis. J. Interferon Cytokine Res. 2006, 26, 71–75.
- Ansari, E.A.; Sahni, K.; Etherington, C.; Morton, A.; Conway, S.P.; Moya, E.; Littlewood, J.M. Ocular signs and symptoms and vitamin A status in patients with cystic fibrosis treated with daily vitamin A supplements. Br. J. Ophthalmol. 1999, 83, 688–691.
- Alghadyan, A.; Aljindan, M.; Alhumeidan, A.; Kazi, G.; Mcmhon, R. Lacrimal glands in cystic fibrosis. Saudi J. Ophthalmol. 2013, 27, 113–116.
- Joshi, D.; Dhawan, A.; Baker, A.J.; Heneghan, M.A. An atypical presentation of cystic fibrosis: A case report. J. Med. Case Rep. 2008, 2, 201.
- Starr, M.R.; Norby, S.M.; Scott, J.P.; Bakri, S.J. Acute retinal vein occlusion and cystic fibrosis. Int. J. Retina Vitreous 2018, 4, 26.
- Gelman, R.; DiMango, E.A.; Schiff, W.M. Sequential bilateral central retinal vein occlusions in a cystic fibrosis patient with hyperhomocysteinemia and hypergamma-globulinemia. Retin Cases Brief Rep. 2013, 7, 362–367.
- Hiscox, R.J.; Purslow, C.; North, R.; Ketchell, I.; Evans, K.S.E. Branch Retinal Vein Occlusion in an Asymptomatic Adult with Cystic Fibrosis. Optom. Vis. Sci. 2014, 91, S52–S54.
- Lass, J.H.; Spurney, R.V.; Dutt, R.M.; Andersson, H.; Kochar, H.; Rodman, H.M.; Stern, R.C.; Doershuk, C.F. A Morphologic and Fluorophotometric Analysis of the Corneal Endothelium in Type I Diabetes Mellitus and Cystic Fibrosis. Am. J. Ophthalmol. 1985, 100, 783–788.
- Abelson, M.B.; Workman, A.; Taylor, A.; Mass, N.A. Eavesdropping on Blepharitis. 2007. Available online: https://www.reviewofophthalmology.com/article/eavesdropping-on-blepharitis (accessed on 7 May 2021).
- Mrugacz, M.; Tobolczyk, J.; Minarowska, A. Retinol binding protein status in relation to ocular surface changes in patients with cystic fibrosis treated with daily vitamin A supplements. Eur. J. Pediatr. 2005, 164, 202–206.
- Vernon, S.A.; Neugebauer, M.A.Z.; Brimlow, G.; Tyrell, J.C.; Hiller, E.J. Conjunctival Xerosis in Cystic Fibrosis. J. R. Soc. Med. 1989, 82, 46–47.
- Brooks, H.L.; Driebe, W.T., Jr.; Schemmer, G.G. Xerophthalmia and Cystic Fibrosis. Arch. Ophthalmol. 1990, 108, 354–357.
- Wamsley, S.; Patel, S.M.; Wood, M.G.; Villalobos, R.; Albert, D.M.; Mootha, V.V. Advanced Keratomalacia with Descemetocele in an Infant With Cystic Fibrosis. Arch. Ophthalmol. 2005, 123, 1012.
- Schupp, C.; Olano-Martin, E.; Gerth, C.; Morrissey, B.M.; Cross, C.E.; Werner, J.S. Lutein, zeaxanthin, macular pigment, and visual function in adult cystic fibrosis patients. Am. J. Clin. Nutr. 2004, 79, 1045–1052.
- Franco, L.G.M.; De Grande, V.; Stella, S.; Reibaldi, M.; Lionetti, E.; Franzonello, C.; Leonardi, S.; Gagliano, C.; Russo, A.; La Rosa, M. Tear Osmolarity in Pediatric Patients with Cystic Fibrosis. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4289.
- Mrugacz, M.M.A.; Bakunowicz-Lazarczyk, A.; Zywalewska, N. Dry eye syndrome in children with cystic fibrosis. Med. Wieku Rozw. 2004, 8, 865–870.
- Modulation of the Magnetic Hyperthermia Response Using Different Superparamagnetic Iron Oxide Nanoparticle Morphologies . Pubmed. Retrieved 2021-8-4
- Successes and Challenges: Inhaled Treatment Approaches Using Magnetic Nanoparticles in Cystic Fibrosis . MPDI. Retrieved 2021-8-4
- Magnetic Nanoparticles as MRI Contrast Agents . Pubmed. Retrieved 2021-8-4
- Magnetic Nanoparticles for Biomedical Purposes: Modern Trends and Prospects . MPDI. Retrieved 2021-8-4
- Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review . MPDI. Retrieved 2021-8-4
- Magnetic Hyperthermia in Y79 Retinoblastoma and ARPE-19 Retinal Epithelial Cells: Tumor Selective Apoptotic Activity of Iron Oxide Nanoparticle . ARVO. Retrieved 2021-8-4
- Metabolic and structural integrity of magnetic nanoparticle-loaded primary endothelial cells for targeted cell therapy . Pubmed. Retrieved 2021-8-4
- Magnetic nanoparticles: a strategy to target the choroidal layer in the posterior segment of the eye. . Europe PMC. Retrieved 2021-8-4
- MRI of Blood Volume and Cellular Uptake of Superparamagnetic Iron in an Animal Model of Choroidal Melanoma . Karger. Retrieved 2021-8-4
- Albumin-based nanostructures for uveal melanoma treatment . Pubmed. Retrieved 2021-8-4
More
