Morphea, also known as localized scleroderma (LoS), comprises a set of autoimmune sclerotic skin diseases. It is characterized by inflammation and limited thickening and induration of the skin; however, in some cases, deeper tissues might also be involved. Although morphea is not considered a life-threatening disease, the apparent cosmetic disfigurement, functional or psychosocial impairment affects multiple fields of patients' quality of life. Therapy for LoS is often unsatisfactory with numerous treatments that have only limited effectiveness or considerable side effects.
- morphea
- localized scleroderma
- laser therapy
1. Introduction
Morphea, also known as localized scleroderma (LoS), encompasses a spectrum of autoimmune cutaneous connective tissue diseases. It is characterized by inflammation, excessive collagen production, and subsequently limited sclerosis of the skin; however, in some cases, deeper tissues such as subcutaneous fat, fascia, muscles, or bones might also be involved. The incidence of morphea is estimated at 27 cases/1,000,000, with greater predominance among females (women are 2.6–6.0 times more frequently affected than men) [1]. According to the German guideline that considers the degree of fibrosis, five types of LoS are distinguished: limited, generalized, linear, deep, and mixed, with the linear subtype predominating in children [2], and circumscribed subtype more common in adults [3]. The etiopathogenesis of LoS has not been fully understood. It is hypothesized to arise as a result of interactions between genetic, epigenetic, environmental, and immunological factors [4].
An active phase of morphea represents limited inflammation, presenting as red or violet patches followed by characteristic porcelain-white or wax-yellow sclerotic plaques, often accompanied by subjective symptoms such as pruritus and/or pain. After several months or years, sclerotic plaques resolve, but skin and/or deeper tissues atrophy and dyspigmentation remain as an atrophic stage. Some severe types, particularly generalized and linear, may be associated with various extracutaneous manifestations [5][6]. It is worth emphasizing that histopathological features mirror the disease phase, although a diagnostic skin biopsy should be taken only in case of an unclear clinical presentation. Early, active lesions present with thickened and homogenized bundles of collagen as well as perivascular infiltrate mostly combined of lymphocytes and plasma cells, alternatively eosinophils and monocytes. The epidermis is usually atrophic. As the disease progresses, homogenization and hardening of collagen appear, which leads to rarefaction of the dermal blood vessels and eccrine glands. The differential diagnosis of LoS should include specific staining: orcein, trichrome, alcian blue, or immunohistochemical for CD34 and factor XIII [5][6].
Clinical evaluation of skin involvement in morphea should be carried out with the use of the ‘localized scleroderma cutaneous assessment tool’ (LoSCAT), that is a standard tool, together with general medical evaluation (Physician’s Global Assessment, PGA) [7]. LoSCAT is a combination of the ‘modified localized scleroderma severity index’ (mLoSSI) and ‘localized scleroderma skin damage index’ (LoSDI). mLoSSI assesses signs of disease severity/activity, including erythema, skin induration, and development of new lesions or expanding pre-existing ones during the last month, whereas LoSDI encompasses dermal and subcutaneous atrophy and pigment changes (hyper-/hypopigmentation) [8].
Appropriate treatment of LoS should be preceded by determining the disease type, activity, severity, and extent of lesions. In mild, limited, and superficial skin lesions, topical treatments (glucocorticoids, calcipotriol, calcineurin inhibitor), or UV phototherapy (UVA1 or PUVA) are sufficient, whereas widespread disease, linear, or deep types require systemic treatment (methotrexate, glucocorticoids). Surgical procedures are primarily dedicated to patients with linear types of LoS, but only in the inactive stage of the disease [3].
Although morphea is not considered a life-threatening disease, the apparent cosmetic disfigurement and functional or psychosocial impairment affect the patients’ quality of life [9]. In this field, medical treatments have often proved unsatisfactory and do not meet patients’ expectations of cosmetic outcome. Thus, it seems obvious that looking for safe and effective practices that correct aesthetic and functional deficits is highly desirable [10][11].
2. Laser Therapy for the Treatment of Morphea
Nowadays, the use of laser devices for the treatment of cosmetic and non-cosmetic skin lesions is increasing, due to the minimally invasive character of treatment reflected in a lower risk of side effects, rapid healing of the targeted skin, and shortened recovery time [12][13][14]. The wide selection of laser devices and the possibility of matching the appropriate mode of action to the clinically observed skin changes is a great advantage [15]. This is particularly essential for LoS, a disease with many clinical faces and an incompletely understood etiology.
We identified a total of twenty relevant studies using laser therapy for LoS. Eight studies were focused on the use of PDL, six on fractional lasers (CO 2 and Er:YAG), four on excimer, and two on either alexandrite or Nd:YAG. The majority of these studies were single case reports or case series. Two studies were investigational [16][17] and one was of observational type [18]. We found only one comparative randomized controlled trial (fractional CO 2 laser vs. UVA-1 therapy) [19]. In all reports, the authors emphasized the efficacy and high safety profile of the applied treatment. It is noteworthy that the therapy was also applied with good response and tolerance in pediatric patients [16]. Undoubtedly, significant variation in study design is evident, including indications, settings of laser parameters, the number of treatments, and the time intervals between them. Moreover, in some studies, laser therapy was used as an additional method to systemic or topical treatment. Therefore, the absolute effect of the laser application in LoS patients could not be assessed. Nonetheless, based on available literature reviewed in this article, and taking into account the mode of action of particular laser devices, we believe that: (i) for active, short-lasting, inflammatory LoS lesions (erythematous plaques), an excimer laser may be suggested, (ii) for active, short-lasting but sclerotic lesions, the use of excimer laser or PDL may be better considered, and on the other hand, (iii) inactive sclerotic lesions as well as hyperpigmented and atrophic ones, can be treated with PDL, alexandrite, Nd:YAG, or fractional lasers ( Figure 13 ).
The latter seems to be a particularly valuable therapeutic option in the case of limb contractures, which are severe complications of the disease, limiting patients’ mobility and reducing the quality of life. In addition, photomechanical fenestrations of the skin (vertical channels of ablation) created by fractional lasers can be used for laser-assisted drug delivery of topically applied formulation, such as PLLA, which can significantly sustain cosmetic improvement in morphea by synergistically increasing new collagen synthesis, resulting in better effectiveness of the applied treatment [19]. However, due to a lack of direct or head-to-head comparative studies, including the use of different types of lasers, case-control studies, or laser therapy against conventional methods (except the study of Shalaby et al. [19]) as well as the small number of patients included in reviewed studies, it is difficult to make clear recommendations. Up to now, there have also been no double-blind controlled trials that would allow validation of the reported effects.
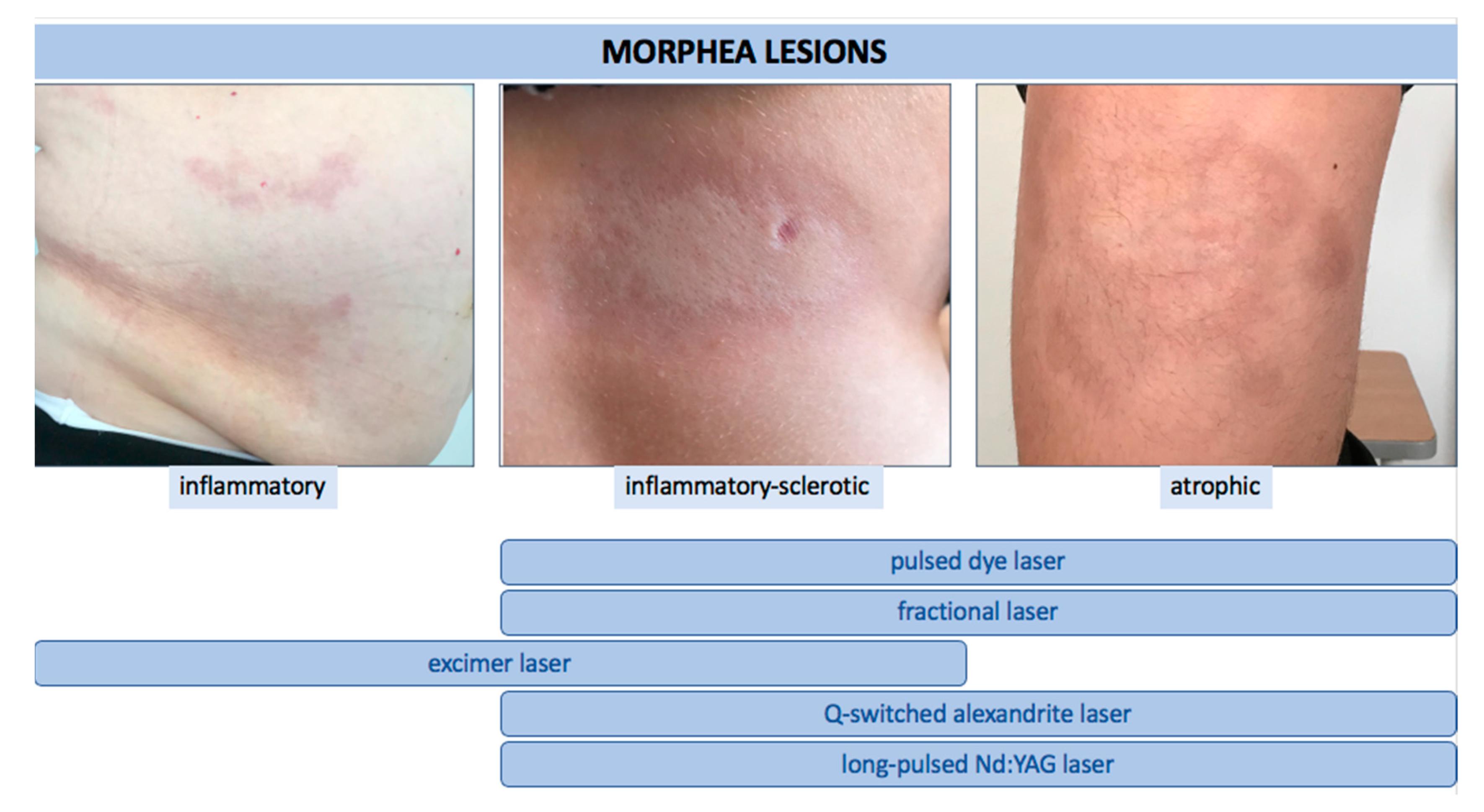 Figure 13. The use of various types of lasers according to clinical stage of LoS.
Figure 13. The use of various types of lasers according to clinical stage of LoS.
There are several previous review articles considering the use of laser treatments in patients with LoS; however, none includes as many studies as ours. According to Zwischenberger et al., 585-nm long-pulse laser therapy is supported by level ≥3 of evidence, meaning there is insufficient evidence to support its efficacy and safety [20]. In the view of Creadore et al., the same level of evidence was obtained for CO 2 fractional laser therapy for morphea-related contractures. On the other hand, level of evidence IB for CO 2 laser treatment was specified for plaque, linear, and en coup de sabre types [11]. Furthermore, Rodríguez-Salgado et al. reported that, according to the Centre for Evidence-Based Medicine, Oxford, fractioned CO 2 laser versus UVA1 is a therapy with the level of evidence 2B and the strength of recommendation B [21].
Laser therapy may be a valuable treatment in some cases of clinically evident LoS lesions or those confirmed by histopathological examination, but it should be kept in mind that this treatment does not slow down the overall course of the disease and does not prevent the formation of new lesions. Therefore, in patients with aggressively progressive lesions, general treatment should be introduced, while laser therapy may be considered as an adjuvant method to treat cutaneous complications of LoS or for cosmetic reasons to provide a better quality of life for the patients. A short recovery time that allows an early return of patients to daily activities, and a single session that enables complete or at least partial remission sustained over a long period of time, certainly form the basis for better patient compliance compared to the systematic use of topical medications [22]. Moreover, in some cases, laser application enables the abandonment of systemic treatment, which may be associated with the occurrence of complications and often requires periodic check-ups. Thus, the effectiveness and safety of laser application should encourage the pursuit of controlled studies with a greater number of patients to better determine its role in the treatment of LoS.
References
- Kreuter, A.; Krieg, T.; Worm, M.; Wenzel, J.; Moinzadeh, P.; Kuhn, A.; Aberer, E.; Scharffetter-Kochanek, K.; Horneff, G.; Reil, E.; et al. German guidelines for the diagnosis and therapy of localized scleroderma. J. Dtsch. Dermatol. Ges. 2016, 14, 199–216.
- Kunzler, E.; Florez-Pollack, S.; Teske, N.; O’Brien, J.; Prasad, S.; Jacobe, H. Linear morphea: Clinical characteristics, disease course, and treatment of the Morphea in Adults and Children cohort. J. Am. Acad. Dermatol. 2019, 80, 1664–1670.e1.
- Knobler, R.; Moinzadeh, P.; Hunzelmann, N.; Kreuter, A.; Cozzio, A.; Mouthon, L.; Cutolo, M.; Rongioletti, F.; Denton, C.P.; Rudnicka, L.; et al. European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, Part 1: Localized scleroderma, systemic sclerosis and overlap syndromes. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1401–1424.
- Wolska-Gawron, K.; Bartosińska, J.; Krasowska, D. MicroRNA in localized scleroderma: A review of literature. Arch. Dermatol. Res. 2020, 312, 317–324.
- Mertens, J.S.; Seyger, M.M.B.; Thurlings, R.M.; Radstake, T.R.D.J.; de Jong, E.M.G.J. Morphea and Eosinophilic Fasciitis: An Update. Am. J. Clin. Dermatol. 2017, 18, 491–512.
- Krasowska, D.; Rudnicka, L.; Dańczak-Pazdrowska, A.; Chodorowska, G.; Woźniacka, A.; Lis-Święty, A.; Czuwara, J.; Maj, J.; Majewski, S.; Sysa-Jędrzejowska, A.; et al. Localized scleroderma (morphea). Diagnostic and therapeutic recommendations of the Polish Dermatological Society. Dermatol. Rev. Przegląd Dermatol. 2019, 106, 333–353.
- Arkachaisri, T.; Vilaiyuk, S.; Torok, K.S.; Medsger, T.A. Development and initial validation of the Localized Scleroderma Skin Damage Index and Physician Global Assessment of disease Damage: A proof-of-concept study. Rheumatology 2010, 49, 373–381.
- Skrzypek-Salamon, A.; Lis-Świȩty, A.; Ranosz-Janicka, I.; Brzezińska-Wcisło, L. Localized Scleroderma Cutaneous Assessment Tool (LoSCAT) adapted for use in adult patients: Report from an initial validation study. Health Qual. Life Outcomes 2018, 16, 1–7.
- Szczȩch, J.; Samotij, D.; Jaworecka, K.; Tobiasz, A.; Reich, A. Quality of Life in Patients with Morphea: A Cross-Sectional Study and a Review of the Current Literature. Biomed. Res. Int. 2020, 2020, 9186274.
- Aksu Arica, D. Cosmetical treatments of connective tissue disorders. Dermatol. Ther. 2019, 32, 10–12.
- Creadore, A.; Watchmaker, J.; Maymone, M.B.C.; Pappas, L.; Lam, C.; Vashi, N.A. Cosmetic treatment in patients with autoimmune connective tissue diseases: Best practices for patients with morphea/systemic sclerosis. J. Am. Acad. Dermatol. 2020, 83, 315–341.
- Rinaldi, F. Laser: A review. Clin. Dermatol. 2008, 26, 590–601.
- Husain, Z.; Alster, T.S. The role of lasers and intense pulsed light technology in dermatology. Clin. Cosmet. Investig. Dermatol. 2016, 9, 29–40.
- Nisticò, S.P.; Tolone, M.; Zingoni, T.; Tamburi, F.; Scali, E.; Bennardo, L.; Cannarozzo, G. A New 675 nm Laser Device in the Treatment of Melasma: Results of a Prospective Observational Study. Photobiomodulation Photomed. Laser Surg. 2020, 38, 560–564.
- Tanzi, E.L.; Lupton, J.R.; Alster, T.S. Lasers in dermatology: Four decades of progress. J. Am. Acad. Dermatol. 2003, 49, 1–34.
- Tawfik, A.A.; Shokir, H.; Soliman, M.; Salah, L.; Fathy, S. Pulsed dye laser in the treatment of localized scleroderma and its effects on CD34+ and factor XIIIa+ Cells: An immunohistochemical study. Am. J. Clin. Dermatol. 2013, 14, 235–241.
- Nisticò, S.P.; Saraceno, R.; Schipani, C.; Costanzo, A.; Chimenti, S. Different applications of monochromatic excimer light in skin diseases. Photomed. Laser Surg. 2009, 27, 647–654.
- Tatu, A.; Radaschin, D.; Constantin, V.; Stana, P.; Ardeleanu, V. Laser therapy in superficial morphea lesions—Indications, limitations and therapeutic alternatives. J. Mind Med. Sci. 2020, 7, 46–51.
- Shalaby, S.M.; Bosseila, M.; Fawzy, M.M.; Abdel Halim, D.M.; Sayed, S.S.; Allam, R.S.H.M. Fractional carbon dioxide laser versus low-dose UVA-1 phototherapy for treatment of localized scleroderma: A clinical and immunohistochemical randomized controlled study. Lasers Med. Sci. 2016, 31, 1707–1715.
- Zwischenberger, B.A.; Jacobe, H.T. A systematic review of morphea treatments and therapeutic algorithm. J. Am. Acad. Dermatol. 2011, 65, 925–941.
- Rodríguez-Salgado, P.; García-Romero, M.T. Morphea: A practical review of its diagnosis, classification and treatment. Gac. Med. Mex. 2019, 155, 483–491.
- Cannarozzo, G.; Negosanti, F.; Sannino, M.; Santoli, M.; Bennardo, L.; Banzola, N.; Negosanti, L.; Nisticò, S.P. Q-switched Nd:YAG laser for cosmetic tattoo removal. Dermatol. Ther. 2019, 32, e13042.
