In vitro cytotoxicity of polymer-carriers, which in the side chains contain the cholinum ionic liquid units with chloride (Cl) or pharmaceutical anions dedicated for antituberculosis therapy, i.e., p-aminosalicylate (PAS) and clavulanate (CLV), was investigated. The carriers and drug conjugates were examined against human bronchial epithelial cells (BEAS-2B) and adenocarcinomic human alveolar basal epithelial cells (A549) as an experimental model cancer cell line possibly coexisting in tuberculosis. The cytotoxicity was evaluated by MTT test and confluency index, as well as by the cytometric analyses, including Annexin-V FITC apoptosis assay. The polymer systems showed supporting activity towards the normal cells and no tumor progress, especially at the highest concentration (100 μg/mL). The analysis of cell death did not show meaningful changes in the case of the BEAS-2B, whereas in the A549 cell line, the cytostatic activity was observed, especially for the drug-free carriers, causing death in up to 80% of cells. This can be regulated by the polymer structure, including the content of cationic units, side-chain length and density, as well as the type and content of pharmaceutical anions. The results of MTT tests, confluency, as well as cytometric analyses, distinguished the polymer systems with Cl/PAS/CLV containing 26% of grafting degree and 43% of ionic units or 46% of grafting degree and 18% of ionic units as the optimal systems.
- graft copolymers
- PIL
- ionic conjugates
- cytotoxicity
- antituberculosis drugs
1. Introduction
2. MTT Cytotoxicity Assay
During the colorimetric assay, due to the mitochondrial reductase, the reaction substrate yellow water-soluble 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) is modified by mammalian cells. The insoluble purple product (E,Z)-5-(4,5-dimethylthiazol-2-yl)-1,3-diphenylformazan (formazan) directly appertains to the number of living cells evaluating a drug system's effect on proliferation [34][35][36][37]. Cell viability assays were performed at a series of concentrations (100–3.125 μg/mL) of nanocarriers I–IV, without pharmaceutical anions and their conjugates with PAS− and CLV− (Figure 1). The inverse relationship of the action on BEAS-2B and A549 cell lines suggests that the compounds are selective for normal and cancer lung cells.
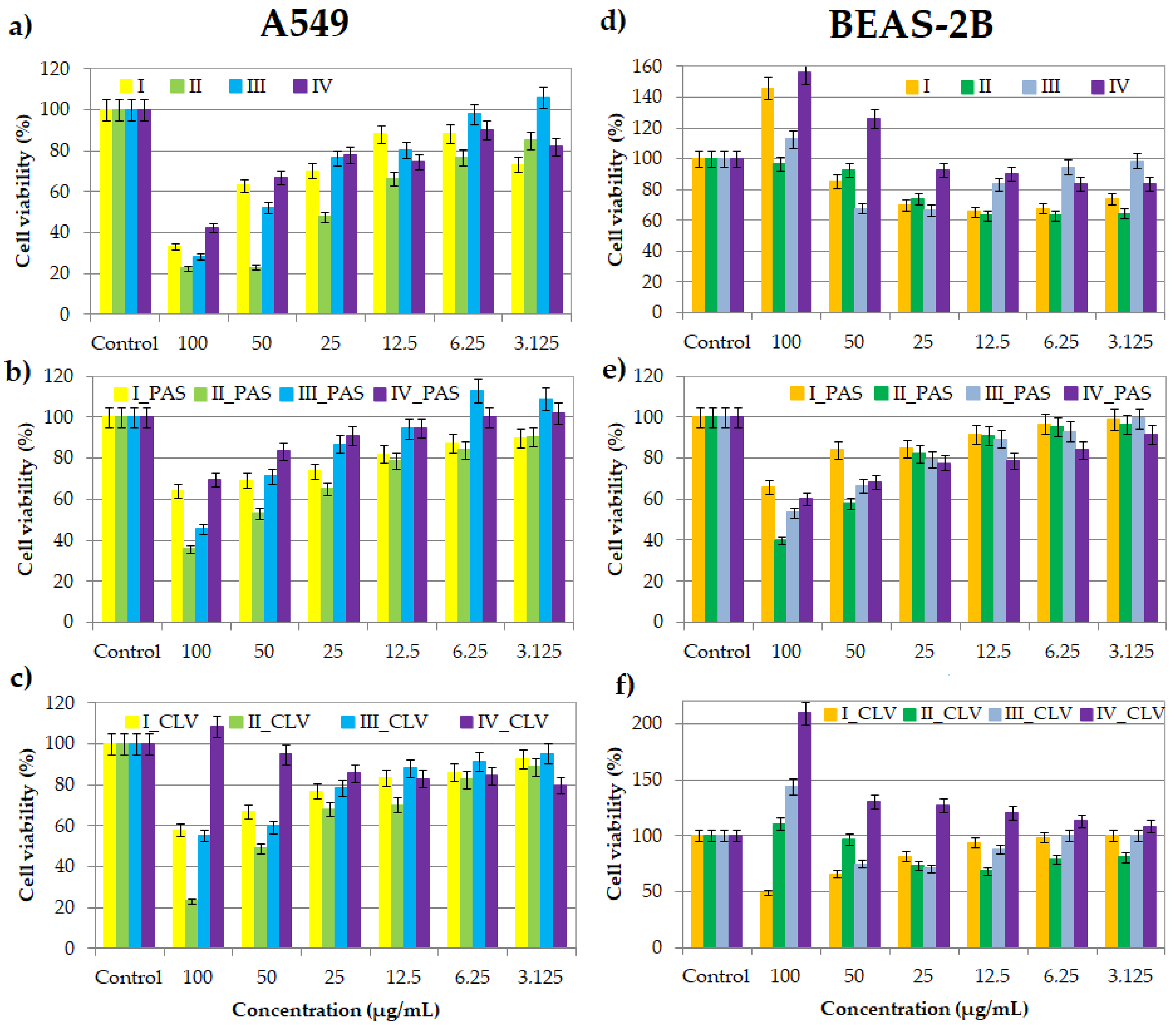 Figure 1. Cell viability of graft copolymers I, II, III and IV (a,d), and their conjugates with PAS (b,e) and CLV (c,f) at different concentrations for treatment of A549 and BEAS-2B cell lines, after 72 h of incubation in comparison to the untreated controls (100%).
Figure 1. Cell viability of graft copolymers I, II, III and IV (a,d), and their conjugates with PAS (b,e) and CLV (c,f) at different concentrations for treatment of A549 and BEAS-2B cell lines, after 72 h of incubation in comparison to the untreated controls (100%).
3. Cytometric Analyses by Flow Cytometry
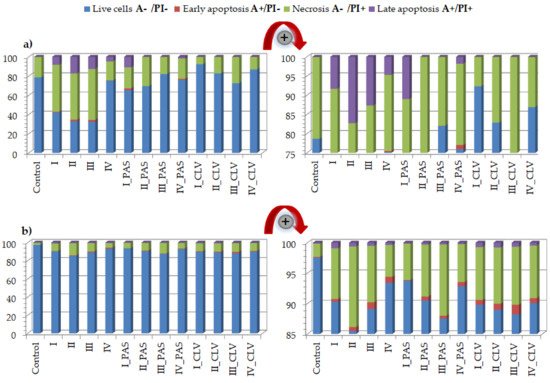
3.1. Apoptosis Assay
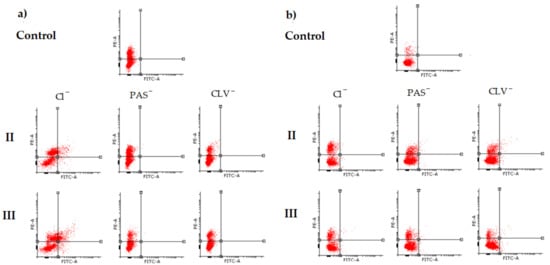
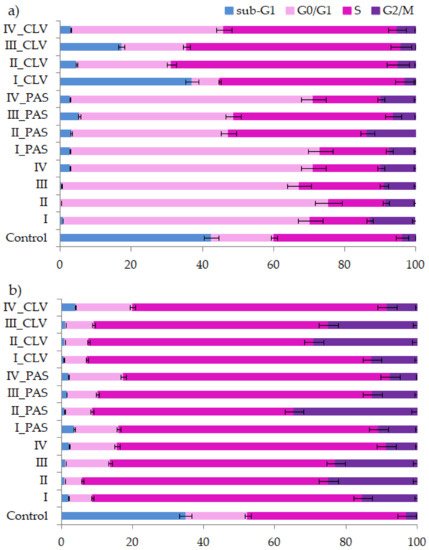
3.2. Cell Cycle Analysis
4. Conclusions
In vitro cytotoxicity evaluation of choline graft, polymer-carriers and their ionic PAS and CLV conjugates was based on MTT, apoptosis assay and cell cycle analysis. These tests indicated a strong correlation between biological action and carrier structure, including the type of attached pharmaceutical anions. Polymer systems with selective activity caused a negative effect on the tumor (A549) cell line, while they did not trigger significant changes in the normal (BEAS-2B) cell line. Moreover, the cytometric analyses proved the specific course of action. During studies on the type of cell death, it was found that in comparison to the control cells, a greater number of A549 cells died, mainly through necrosis. In turn, these compounds had no meaningful impact on BEAS-2B cells. Additional confirmation was achieved by cell cycle determination. Such findings suggest the potential usage of novel drugs for respiratory system diseases because of a wide application against cancer cells or pathogens (originated structures were reported as antimicrobial). For further findings, the specific test for antituberculosis therapy using standard assays should be performed [42]. The investigated ionic graft copolymers and their conjugates, previously tested for physicochemical evaluation, and evaluated cytotoxicity in this report, fulfilled the basic criteria for drug delivery systems. They are promising carriers of ionic drugs, especially those with a higher content of ionic units at lower graft density or lower content of ionic units at higher graft density, which can be used in the future for the treatment of lung diseases, such as tuberculosis, given the required specialized biomedical assessments.References
- Amstad, E.; Reimhult, E. Nanoparticle actuated hollow drug delivery vehicles. Nanomedicine 2000, 7, 145–164.
- Neuse, E.W. Synthetic Polymers as Drug-Delivery Vehicles in Medicine. Met. Based Drugs 2008, 2008, 1–19.
- Deb, P.; Kokaz, S.; Abed, S.; Paradkar, A.; Tekade, R. Pharmaceutical and Biomedical Applications of Polymers. In Advances in Pharmaceutical Product Development and Research, Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 203–267.
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173.
- Kopeček, J. Polymer–drug conjugates: Origins, progress to date and future directions. Adv. Drug Deliv. Rev. 2013, 65, 49–59.
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037.
- Khandare, J.; Minko, T. Polymer–drug conjugates: Progress in polymeric prodrugs. Prog. Polym. Sci. 2006, 31, 359–397.
- Mamontova, N.V.; Chernyak, E.I.; Amosov, E.V.; Gatilov, Y.V.; Vinogradova, V.I.; Aripova, S.F.; Grigor’ev, I. First Ionic Conjugates of Dihydroquercetin Monosuccinate with Amines. Chem. Nat. Compd. 2017, 53, 1045–1051.
- Saraswat, J.; Wani, F.A.; Dar, K.I.; Rizvi, M.M.A.; Patel, R. Noncovalent Conjugates of Ionic Liquid with Antibacterial Peptide Melittin: An Efficient Combination against Bacterial Cells. ACS Omega 2020, 5, 6376–6388.
- He, D.; Liu, Z.; Huang, L. Progress in Ionic Liquids as Reaction Media, Monomers and Additives in High-Performance Polymers. In Solvents, Ionic Liquids and Solvent Effects; Glossman-Mitnik, D., Ed.; IntechOpen: London, UK, 2020; pp. 99–124.
- Eftekhari, A.; Saito, T. Synthesis and properties of polymerized ionic liquids. Eur. Polym. J. 2017, 90, 245–272.
- Bielas, R.; Mielańczyk, A.; Skonieczna, M.; Mielańczyk, Ł.; Neugebauer, D. Choline supported poly(ionic liquid) graft copolymers as novel delivery systems of anionic pharmaceuticals for anti-flammatory and anti-coagulant therapy. Sci. Rep. 2019, 9, 14410.
- Fedotova, M.V.; Kruchinin, S.E.; Chuev, G.N. Features of local ordering of biocompatible ionic liquids: The case of choline-based amino acid ionic liquids. J. Mol. Liq. 2019, 296, 112081.
- Lin, X.; Yang, Y.; Li, S.; Song, Y.; Ma, G.; Su, Z.; Zhang, S. Unique stabilizing mechanism provided by biocompatible choline-based ionic liquids for inhibiting dissociation of inactivated foot-and-mouth disease virus particles. RSC Adv. 2019, 9, 13933–13939.
- Petkovic, M.; Ferguson, J.L.; Gunaratne, H.Q.N.; Ferreira, R.; Leitão, M.C.; Seddon, K.R.; Rebelo, L.P.N.; Pereira, C.S. Novel biocompatybile cholinum-based ionic liquids-toxicity and biodegradability. Green Chem. 2010, 12, 643–649.
- Noshadi, I.; Walker, B.W.; Portillo-Lara, R. Engineering Biodegradable and Biocompatible Bio-ionic Liquid Conjugated Hydrogels with Tunable Conductivity and Mechanical Properties. Sci. Rep. 2017, 7, 4345.
- Isik, M.; Gracia, R.; Kollnus, L.C.; Tomé, L.C.; Marrucho, I.M.; Mecerreyes, D. Cholinium-Based Poly(ionic liquid)s: Synthesis, Characterization, and Application as Biocompatible Ion Gels and Cellulose Coatings. ACS Macro Lett. 2013, 2, 975–979.
- Md Moshikur, R.; Chowdhury, M.R.; Moniruzzaman, M.; Goto, M. Biocompatible ionic liquids and their application in pharmaceutics. Green Chem. 2020, 22, 8116–8139.
- Ibsen, K.N.; Ma, H.; Banerjee, A.; Tanner, E.E.L.; Nangia, S.; Mitragotri, S. Mechanism of Antibacterial Activity of Choline-Based Ionic Liquids (CAGE). ACS Biomater. Sci. Eng. 2018, 4, 2370–2379.
- Yuan, J.; Soll, S.; Drechsler, M.; Müller, A.; Antonietti, M. Self-Assembly of Poly(ionic liquid)s: Polymerization, Mesostructure Formation, and Directional Alignment in One Step. J. Am. Chem. Soc. 2011, 133, 17556–17559.
- Guo, J.; Zhou, Y.; Qiu, L.; Yuan, C.; Yan, F. Self-assembly of amphiphilic random co-poly(ionic liquid)s: The effect of anions, molecular weight, and molecular weight distribution. Polym. Chem. 2013, 4, 4004.
- Hosseinzadeh, F.; Mahkam, M.; Galehassadi, M. Synthesis and characterization of ionic liquid functionalized polymers for drug delivery of an anti-inflammatory drug. Des. Monomers Polym. 2012, 15, 279–388.
- Gao, Y.; Arritt, S.W.; Twamley, B.; Shreeve, J.M. Guanidinium-Based Ionic Liquids. Inorg. Chem. 2005, 44, 1704–1712.
- Stenzel, M.; Barner-Kowollik, C.; Davis, T.; Dalton, H.M. Amphiphilic Block Copolymers Based on Poly(2-acryloyloxyethyl phosphorylcholine) Prepared via RAFT Polymerisation as Biocompatible Nanocontainers. Macromol. Biosci. 2004, 4, 445–453.
- Yu, Y.; Yao, Y.; van Lin, S.; de Beer, S. Specific anion effects on the hydration and tribological properties of zwitterionic phosphorylcholine-based brushes. Eur. Polym. J. 2009, 112, 222–227.
- Joubert, F.; Yeo, R.; Sharples, G.; Musa, O.M.; Hodgson, D.; Cameron, N. Preparation of an Antibacterial Poly(ionic liquid) Graft Copolymer of Hydroxyethyl Cellulose. Biomacromolecules 2015, 16, 3970–3979.
- Niesyto, K.; Neugebauer, D. Synthesis and Characterization of Ionic Graft Copolymers: Introduction and In Vitro Release of Antibacterial Drug by Anion Exchange. Polymers 2020, 12, 2159.
- Niesyto, K.; Neugebauer, D. Linear Copolymers Based on Choline Ionic Liquid Carrying Anti-Tuberculosis Drugs: Influence of Anion Type on Physicochemical Properties and Drug Release. Int. J. Mol. Sci. 2021, 22, 284.
- Gorbunova, M.; Lemkina, L.; Borisova, I. New guanidine-containing polyelectrolytes as advanced antibacterial materials. Eur. Polym. J. 2018, 105, 426–433.
- Shekaari, H.; Zafarani-Moattar, M.; Mirheydari, S.; Agha, E. The effect of pharmaceutically active ionic liquids, 1-methyl-(3-hexyl or octyl) imidazolium Ibuprofenate on the thermodynamic and transport properties of aqueous solutions of glycine at T = 298.2 K and p = 0.087 MPa. J. Mol. Liq. 2019, 288, 111009.
- Lu, B.; Zhou, G.; Xiao, F.; He, Q.; Zhang, J. Stimuli-Responsive Poly(ionic liquid) Nanoparticle for Controlled Drug Delivery. J.Mater. Chem. B 2020, 8, 7994–8001.
- Bielas, R.; Siewniak, A.; Skonieczna, M.; Adamiec, M.; Mielańczyk, Ł.; Neugebauer, D. Choline based polymethacrylate matrix with pharmaceutical cations as co-delivery system for antibacterial and anti-inflammatory combined therapy. J. Mol. Liq. 2019, 285, 114–122.
- De Jong, W.; Borm, P. Drug delivery and nanoparticles: Application and hazards. Int. J. Nanomed. 2008, 3, 133–149.
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, 6.
- Bahuguna, A.; Khan, I.; Bajpai, V.K.; Kang, S.C. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh. J. Pharmacol. 2017, 12, 115–118.
- Bopp, S.K.; Lettieri, T. Comparison of four different colorimetric and fluorometric cytotoxicity assays in a zebrafish liver cell line. BMC Pharmacol. 2008, 8, 8.
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. Methods Mol. Biol. 2017, 1601, 1–17.
- Darzynkiewicz, Z.; Bedner, E.; Smolewski, P. Flow cytometry in analysis of cell cycle and apoptosis. Semin Hematol. 2001, 38, 179–193.
- Ormerod, M.G. Investigating the relationship between the cell cycle and apoptosis using flow cytometry. J. Immunol. Methods 2002, 265, 73–80.
- Koopman, G.; Reutelingsperger, C.P.M.; Kuijten, G.A.M.; Keehnen, R.M.J.; Pals, S.T.; Van Oers, M.H.J. Annexin V for Flow Cytometric Detection of Phosphatidylserine Expression on B Cells Undergoing Apoptosis. Blood 1994, 84, 1415–1420.
- Riccardi, C.; Nicoletti, I. Analysis of Apoptosis by Propidium Iodide Staining and Flow Cytometry. Nat. Protoc. 2006, 1, 1458–1461.
- Akcali, S.; Surucuoglu, S.; Cicek, C.; Ozbakkaloglu, B. In vitro activity of ciprofloxacin, ofloxacin and levofloxacin against Mycobacterium tuberculosis. Ann. Saudi Med. 2005, 25, 409–412.
