Colitis-complex diarrhea (CCD) in pigs can be defined as a type of diarrhea, which is associated with colonic inflammation and disrupted colonic gut barrier functionality as well as infiltration of neutrophils at the inflamed colon in growing pigs (4–16 weeks post-weaning). It is a challenge for the pig industry as it is associated with the high use of antibiotics, reduced animal welfare, and depressed growth rate. The exact etiology of CCD is still unclear; however, pathogens including Brachyspira (B.) hyodysenteriae, B. pilosicoli, and swine whipworms such as Trichuris (T.) suis have been involved in specific colitis (SC). In the absence of specific pathogens, dietary factors, such as high levels of protein, pelleted feedstuffs, and lack of sufficient antioxidants, can result in non-specific colitis (NSC). On the other hand, supplement of polyunsaturated fatty acids (PUFA) and polyphenols, sufficient supply of essential amino acids (e.g., threonine, cysteine, and proline), short-chain fatty acids (SCFA; especially butyrate), and resistant starch have shown to confer preventing/ameliorating effects on CCD.
- colonic inflammation
- adult pigs
- biomarkers
- dietary strategies
- specific colitis
- non-specific colitis
1. Colonic Epithelium Function
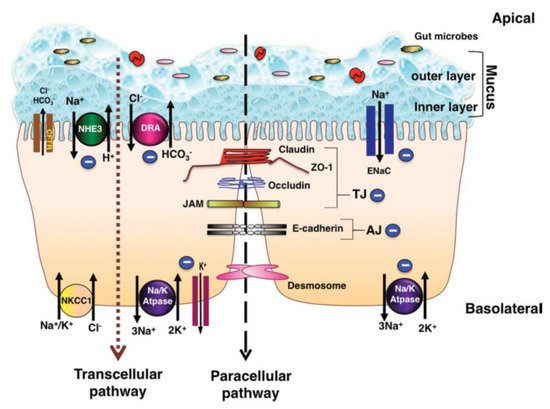
1.1. Colonic Ion Exchange
The pathogenesis of diarrhea related to inflammation in the colon is a multifactorial event
. Understanding the ion-absorption/secretion mechanisms in the colon and the effects of inflammatory mediators on epithelial transport function is therefore of great importance.
depicts the major ion transportation pathways in the colon. Generally, normal stool is low in Cl
and Na
and high in K
since reabsorption of Na
and secretion of K
(both active and passive) takes place in the colon
. The reason for the low level of Cl
in normal stool is that short-chain fatty acids (SCFA) produced in the colon can replace Cl
, while luminal concentration of HCO
is similar to its concentration in plasma
. In the colon, Na
gets absorbed through the stimulatory effect of SCFA, through an aldosterone-sensitive sodium absorption by the epithelial Na
channel (ENaC) in the distal colon
, and through an Na-H exchange parallel to a Cl-HCO
exchange (in the proximal colon) which is responsible for an electro-neutral Na-Cl absorption
. SCFA are the primary anions in the lumen, which also contribute to Na absorption through apical Na-H, Cl-SCFA, and SCFA-HCO
exchanges; this type of Na
absorption is not hampered by cyclic adenosine monophosphate (cAMP)
. Secondary messengers, such as cAMP, cyclic guanosine monophosphate (cGMP), intracellular calcium (Ca
), and neurohumoral substances, can activate Na-H exchange (NHE) genes such as NHE3, which is involved in neutral absorption of sodium
. However, increased mucosal cAMP and intracellular Ca
concentration in the colon can inhibit apical Na-H and Cl-HCO
exchanges and reduce absorption of Na
and Cl
, consequently reducing the water absorption
. For apical exchange of Cl-HCO
in the colon, downregulation in adenoma (DRA or SLC26A3) is the major exchanger, which is a chloride-sulfate anion transporter in the upper crypt and surface epithelium of the colon
. DRA activity was shown to be inhibited by increased cellular cAMP and Ca
. Moreover, the gene expression of DRA was reported to be heavily diminished in colonic inflammation as an effect of the interleukin-1β (IL-1β) cytokine, hence hampering Cl
absorption
.
In animal studies, it has been shown that some bacterial pathogens, such as
,
toxin type 1
, and
increase intracellular concentrations of Ca
, resulting in inhibition of NHE3 and stimulation of an excessive secretion of Na
and Cl
. Therefore, impaired Na
absorption and stimulation of Ca
secretion can result in diarrhea
. Excessive intracellular secretion of Ca
could be a tertiary effect of microbial pathogenesis since pathogens first stimulate the enteric nervous system, then increase the release of neurotransmitters and, ultimately, enhance the secretion of Ca
. Increased levels of luminal ions can be expected as a result of inhibition of Na
and Cl
absorption along with stimulation of excessive Cl
secretion after disruption of the colonic mucus layer by either pathogen- and/or feed-induced inflammatory mediators
. Quantification of recovered Cl
from fecal samples
of affected pigs can potentially be an indicator of an inflamed colonic epithelium of pigs with colitis.
1.2. Cytokines and Luminal Function
Cytokines are small peptide molecules acting as important mediators in the regulation of the immune and inflammatory responses produced by epithelial cells, endothelial cells, and fibroblasts
. Cytokines such as interferon-γ (IFN-γ) can directly alter the epithelial tight junctions and increase the transepithelial permeability
. In an inflamed colon, cytokines, such as IL-1β
, IFN-γ, and tumor necrosis factor-α (TNF-α)
, are culprit keys involved in perturbing the absorption of Na
and Cl
and consequently the water absorption
, giving rise to stool water content. Overexpression of these cytokines in the colonic mucosa can also cause mucosal damage and dysfunction, which may lead to diarrhea
. The increased ionic level in the lumen can reciprocally disturb the absorptive/secretive balance and cause diarrhea in two distinct ways: (1) by increasing the further secretion of ions upon the induced imbalanced electrical charge, and (2) by increasing the extracellular osmotic pressure, resulting in more water diffusion from the enterocytes.
Aside from ion transportation, the intestinal epithelium performs a barrier function through enterocytes and their encircling tight junctions (zona occludens), which limits the passive flow of luminal contents into the blood and lymphatics and the other way around
. The restriction of tight junctions is higher in the colon vs. small intestine, and this restriction increases from the proximal to the distal colon
. In human patients with Crohn’s disease (CD), the epithelium in inflamed intestinal segments is characterized by a reduction of tight junction strands, strand breaks, and alterations of tight junction protein content and composition
. In patients with ulcerative colitis (UC), micro-erosions caused by upregulated epithelial apoptosis as well as a remarkable increase in claudin-2 are the main reasons for early epithelial leaks
. The mucosal inflammation in UC increases the permeability of colonic epithelium by changing the tight junctions, which could be a contributing reason to diarrhea appearing due to colonic inflammation
.
1.3. Mucins
In addition to the epithelial integrity and the presence of commensal bacteria, the non-immune intestinal barrier is due to mucus production
. Mucins are gel-forming high-molecular-weight glycoproteins, which are synthesized and secreted by goblet cells and also act as an important feature for gut barrier functionality
. In the intestinal tract, MUC2 is the predominant secretory mucin, and it is the main structural component of the colonic mucus layer
. Perturbed synthesis and secretion of mucin by the goblet cells, in response to pathophysiological alterations in the intestinal mucosa, result in changes in the mucus gel
. In the absence of inflammation, the mucus layer is about 700 μm thick
, whereas exposure to specific bioactive factors, such as hormones, inflammatory mediators, and microbial factors, such as lipopolysaccharides (LPS), flagellin A, and lipoteichoic acids, can upregulate mucin production, in particular MUC2
. Upregulation of mucins such as MUC5AC and MUC4 along with mucus hypersecretion of goblet cells were also shown to be associated with inflammatory diseases of the epithelium
.
2. Colitis
Inflammation is a constant ongoing process of a normally functioning colon as the healthy colon is continuously offsetting inflammatory responses as a result of exposure to a wide variety of, for example, bacteria in the lumen, dietary antigens, and toxins
. If the inflammatory responses go beyond a point to jeopardize the luminal integrity, it can result in diarrhea by increasing gut permeability
and impairing its absorptive functionality
. Colitis is referred to as inflammation in the colon, which is a consequence of a complex biological defense mechanism against harmful stimuli such as pathogenic bacteria and physical damages
. Diarrhea is a non-specific sign of colitis occurring in the acute phase of colitis
and for pigs commonly referred to as CCD. This type of diarrhea is typically non-hemorrhagic (in severe cases, hemorrhagic) mucoid diarrhea and infiltration of neutrophils (
) in the inflamed colon of growing pigs (4–16 weeks post-weaning)
.
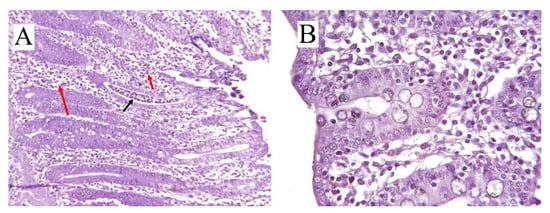
(
) Cross-sectional histology (hematoxylin/eosin-stained) of inflamed colon in pig (8 weeks old) with ×10 scale of magnification. Infiltration of neutrophils (black arrow) and mononuclear cells (red arrows) in the crypts can be seen. (
) Colonic cross-sectional histology of a healthy pig (11 week) with ×25 scale of magnification.
However, in younger pigs (e.g., during nursery and post-weaning stage), the main reason for diarrhea is enterotoxigenic
(
)
(ETEC) with fimbriae types F4 and F18
. CCD occurs if the epithelia’s barrier function is compromised by loss of epithelial cells or disruption of tight junctions (
); in that scenario, hydrostatic pressure in blood vessels and lymphatics will cause water and electrolytes, mucus, protein, and in some cases, red and white cells to accumulate luminally
. The bottleneck of colitis diagnosis is the examination of postmortem lesions and histological test (hematoxylin and eosin staining) on the colonic epithelium (
)
. In the case of acute or chronic inflammation or necrosis of the colonic mucosa, exudative diarrhea occurs, which is characterized by an increase in fluid production, excretion of the inflammatory products such as serum proteins, and a reduction in absorption of fluids and electrolytes
. Unlike the small intestine, the colonic absorptive function is related to the epithelial intercrypt surface instead of villi, while the immature epithelial cells of the crypts perform the secretory function as in the small intestine
.
2.1. Specific Colitis
The most important etiology and epidemiology of common forms of SC (i.e., when one or more pathogens are involved) are briefly presented in
, which will be discussed in the following sections.
Etiology and epidemiology of CCD in growing pigs according to the causative factors and mechanisms of action.
| Type of Colitis | Causative Factor | Affected Site | Pigs Age, Week | Mechanism of Action | Clinical Signs | Gross Lesion | References |
|---|
| SC | |||||||
| Swine dysentery | B. hyodysenteriae | Cecum and colon | 6–18 | Absorptive dysfunctionality, hemolysins, and degradative enzymes | Loose stool, mucoid, hemorrhagic diarrhea, dehydration, and retarded growth rate | Inflamed epithelium, mucosal damage, hyperplasia of the crypts, and spirochetal attachment | [31][32][33][34] |
| Spirochetal colitis | B. pilosicoli | Cecum and colon | 4–20 | Absorptive dysfunctionality | Mild non-hemorrhagic, mucoid diarrhea, retarded growth rate | Inflamed epithelium, moderate catarrhal colitis, flaccid and thin luminal wall, appearance of small adherent nodules of digesta | [9][35][33][34][36] |
| Parasitic colitis | T. suis | Cecum and spiral colon | 4–10 | Stimulation of the epithelium and cascading inflammatory responses by hatched eggs and adult worms | Dark loose stool, mucoid to hemorrhagic diarrhea, dehydration, anorexia, and increased feed conversion ratio | Crypt hyperplasia, goblet cell hyperplasia, a general hypertrophy of mucosa, and presence of bipolar eggs | [37][38][39][40][41] |
| NSC | Dietary factors | Cecum and colon | 4–12 | Absorptive dysfunctionality and increased epithelial permeability | Loose and mucoid, non-hemorrhagic diarrhea | Mucosal hyperplasia, mononuclear cell infiltration, multifocal mucosal erosions, increased crypt depth | [33][23][30][42] |
2.1.1. Swine Dysentery
Swine dysentery (SD) is a severe mucohemorrhagic diarrheal colitis caused by
formerly known as
and
, a Gram-negative and flagellated anaerobic spirochete
. Together, the abundance of goblet cells (as a matrix and source of nutrients) and the anaerobic environment in the large bowel contribute to a favorable environment for
, confided to the colon
. Colonization of the large intestine (cecum and colon) by
results in lysis of mucosal cells by bacterial virulence factors, such as hemolysins and proteases, degradative enzymes, and inflammation
. Bacterial proteases were reported to be involved in malabsorption of carbohydrates by inactivating the oligosaccharidases of the brush border
, and the damages to the intestinal wall could aid proliferation of other pathogens
. The incidence of SD occurs in pigs during 6–18 weeks of age
, and the first clinical symptom is excretion of watery or semisolid feces, which progresses as diarrheal feces with large amounts of mucus and variable amounts of blood
. Physiologically, SD is associated with dehydration, acidosis, hyperkalemia and, in severe cases, death
. Nonetheless, the apparent prevalence of
in Danish herds was shown to be relatively low: 2.5%
.
is associated with producing a cytotoxic hemolysin as a virulence determinant, which, concurrent with other anaerobic colonic bacteria such as
or
, can synergize the development of SD in growing pigs
. The colonic goblet cells of pigs with severe clinical signs of SD may show a substantial increase in the levels of MUC2 mucin and de novo expression of MUC5AC mucin and increased
binding ability to the epithelial mucus
. This indicates that morphological changes such as increased mucin production in the colon induced by
are mechanisms to facilitate the further infection by increasing the binding sites for
attachment. Therefore, increased expression of MUC2 in the inflamed colon of pigs and subsequent higher levels in feces may potentially be considered as contributing biomarkers for the diagnosis of colonic inflammations (
). The consequent increased turnover rate of intestinal mucosal cells could be a reason for increased crypt depth
observed in pigs with colitis
. However, the genes encoding MUC2 were remarkably downregulated in crypt cells of pigs infected with
, an obligate intracellular, Gram-negative, and microaerophilic pathogen, which infects cells of the ileal epithelium (small intestine)
. Therefore, characterization of the variations in mucin expression of the pig colon may be considered as a diagnostic tool for inflammatory diseases such as colitis and discriminating ileitis from colitis.
Summary of non-invasive putative biomarkers associated with colonic inflammation.
| Biomarkers | Type | Direction | Recovery Site | Causative Factor | Affected Site | Reference |
|---|
| MUC2 and MUC5AC | Mucin | Increased expression | Feces | Colitis, swine dysentry | Large intestine | [17][21][22] |
| LPS 1 | Saccharide | Increased expression | Serum | Gram-negative pathogens, e.g., B. hyodysenteriae | Small and large intestine | [48][49][50][ |
, and iron is required to be in adequate amounts. Urinary excretion of N-methylnicotinamide is a reliable indicator for nicotinic acid deficiency
. It was reported that sufficient dietary supplementation of niacin (≥15 µg/g feed) could prevent the incidence of NSC and also reverse the condition in pigs
. Niacin inhibits inflammation responses by downregulating nuclear transcription factors-κB (NTF-κB,
) signaling pathway in guinea pigs
and was shown to reverse colitis in rats challenged by iodoacetamide
. Other dietary factors involved in the incidence of NSC could be dietary protein, dietary fiber, and pelleted feedstuff, which will be discussed below.
| Factor | Level | Effect | Reference |
|---|
| Trypsin inhibitor | High | Increased undegraded protein in large intestine and inflammation and causing NSC 1 | [33] | |||
| 51 | ] | |||||
| Calprotectin and lactoferrin | Protein | Increased expression | Feces and serum | Colitis and inflammatory factors | Large intestine | [52][53][54][55] |
| Na+, Cl−, HCO3-, and K+ | Ion | Reduced absorption and increased luminal accumulation | Feces | B. hyodysenteriae | Large intestine | [9][5][12][44] |
| TNF-α 2, IFN-γ 3, IL-1β 4, IL-6 5, and IL-10 6 | Cytokines | Increased expression | Serum and mucus | Pathogens | Small and large intestine | [1][5][12][23][50][56] |
| NF-κB 7 | Protein | Increased expression in macrophages and in epithelial cells | Serum | IL-1β and TNF-α, LPS, and ROS 8 | Epithelial cells of inflamed colon | [51][56][57][58][59] |
| CRP 8, HP 9, and pig-MAP 10 | Protein | Increased concentration | Serum | LPS, IL-1β, and TNF-α | Epithelial cells of colon, and hepatic cells | [50] |
| FRAP 11, TBARS 12, and ROS 13 | TAC 14 assay | Increased expression | Serum | Oxidative stress | Epithelial cells of colon | [60][61] |
| TEAC 15, CUPRAC 16, AOPP 17, and H2O2 | TAC assay | Increased expression | Saliva | Oxidative stress | Epithelial cells of colon | [61][62] |
An indicator of infectious diarrhea is the presence of secretory and pro-inflammatory mediators, such as histamine, prostaglandins, and 5HT (5-hydroxytryptamine or serotonin) as well as proteases released from the mast cells
. However,
can be involved in diarrhea by causing mucosal inflammation in the large intestine upon its attachment, malabsorption by impairing Na
, and water-absorptive functionality of the colon epithelium
, resulting in an increased accumulation of water, Na
, Cl
, HCO
, and K
in the luminal fluid
. As a consequence of disrupted absorptive functionality, acidosis and increased serum’s K
could potentially be used as a diagnostic factor in SC
. In addition, Cl
is a key-secreted ion that facilitates the transmucosal movement of both Na
and water, which is defused across the paracellular space and into the lumen under the effect of electrical and osmotic gradient
. Since glucose, galactose, and amino acid are co-transported with Na
into the colonocytes, their luminal accumulation as a result of SD is expected. Consequently, due to an osmotic gradient caused by undigested carbohydrates
in the lumen, more water diffusion could be anticipated; hence, exacerbated diarrhea in infected pigs can occur. This mode of action was attributed to
in addition to the fact that it has no pernicious effects on the intestinal secretory processes and solely disrupts the absorptive function of the epithelium
.
According to Carr et al.
, a noteworthy difference between the pathogenic etiologies of colitis is that
is a strict anaerobe, occurring only in the large bowel (cecum and colon); therefore, the associated lesions of SD are not seen in the small intestine. In this regard, Argenzio
showed that the kinetics of glucose-stimulated water absorption were identical between infected and healthy pigs, indicating that SD had no disruptive effect on the small intestine and ileum. Therefore, oral glucose-electrolyte rehydration would be a pragmatic approach in restoring extracellular fluid losses associated with SD
. Glucose and amino acids are co-transported with Na
, and the existing intracellular Na
gradient is a driving force for amino acids, oligopeptides, and sugar absorption with the following water absorption due to the osmotic pressure, hence rehydration
.
2.1.2. Spirochetal Colitis
Spirochetal colitis or porcine colonic spirochetosis affects pigs aged 4–20 weeks, and the clinical signs commonly appear 10–14 days after mixing and changing the feed to a grower or pelleted diet
.
is the causative pathogen of spirochetal colitis
with the same mode of action as
on the absorptive function of the colonocytes, albeit histopathologic lesions are less severe with milder non-hemorrhagic diarrhea
.
causes inflammation and mucosal damage throughout the cecum and colon (typhlocolitis;
), which by reducing the surface area of the large intestine available for absorption causes diarrhea
. Gross lesions of spirochetal colitis are associated with diarrhea with loose to watery and in some cases mucoid feces, and mild mucosal reddening and flecks of pus, resulting in diarrhea and reduced growth rate
. Microscopic signs of this disease could be seen as mild to moderate catarrhal colitis with erosions of the surface epithelium and spirochetal colonization of the colon epithelium
. Trott et al.
characterized the postmortem signs of
infection as increased size of the colon in infected animals with flaccid and thin wall, filled with watery, slightly mucoid content, and the mucosal surface was covered with small adherent nodules of digesta.
The occurrence of this disease was characterized by the coinfection of the protozoa
associated with the mucosa and lamina propria along with
attached by one end to the colonic epithelium, which triggers neutrophilic exocytosis, excess mucus and increased crypt cell mitotic index
. A study in 79 Danish pig herds showed an apparent prevalence of
to be 19.0%
. In case of SC, the presence of microbially derived LPS in serum could be a reliable biomarker for microbial detections (
) since LPS is a part of the outer membrane of Gram-negative bacteria and induces inflammatory responses
.
LPS contain the lipid A-sugar core of approx. 10–16 kDa, which is the main difference from LPS of other species, and these lipopolysaccharides produced by
are speculated to be involved in the colonic damage
. In an in vivo study, pigs administered with LPS developed acute phase response (APR) and hepatic production of acute phase proteins, e.g., C-reactive protein (CRP), haptoglobin (HP), and pig major acute phase protein (pig-MAP) as a result of increased stimulation of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6
. Therefore, in case of SC, the seral measurement of LPS, CRP, HP, and pig-MAP could also be considered as putative biomarkers.
2.1.3. Parasitic Colitis
Swine whipworms can cause inflammation in the cecum and spiral colon, resulting in defecation of dark loose stools containing blood and diarrhea
. In Danish organic pig farms, with access to pastures, there is a high risk of the exposure of the animal to the parasite eggs, present mainly in the pasture
. Among swine parasitic whipworms,
can infect young and growing pigs in both indoor and outdoor production systems
that can cause severe mucoid to hemorrhagic diarrhea, dehydration, anorexia, reduced gain, and increased feed conversion ratio
. The eggs of the parasite are passed in feces from infected animals, and, once eaten, they can travel through the digestive tract, and in the small intestine and cecum, the larva hatches and penetrates the mucosa through the crypts of Lieberkühn in the distal ileum, cecum, and colon
. Crypt hyperplasia, goblet cell hyperplasia, and a general hypertrophy of mucosa in the proximal colon in addition to the increased level of parasite-specific antibodies in the serum of pigs infected with
in week 10 of age were reported to be associated with
infection
. Adult worms are highly potent to cascade an inflammatory response in addition to inducing immunosuppressive properties in infected pigs through high release of nitric oxide (NO) and upregulating arginase activity in macrophages
. The excessive production of NO in abnormal situations induces inflammation in the colon
.
Conducting clinical serologic assays for the increased level of 20 kDa excretory/secretory glycoprotein antigen in the serum of infected pigs could be used for diagnosis of
. Hygienic practices to prevent the fecal-oral transmission of the eggs, including regular removal of feces and organic debris, could be considered as preventive strategies, and, once infected, pigs could be treated with anthelmintic
.
2.2. Non-Specific Colitis and Role of Dietary Factors in Inducing Colitis
When no specific pathogens can be identified, the term NSC is designated to describe this form of colitis
. As shown in
, NSC mainly affects growing pigs (12–40 kg, approx. 4–12 weeks post-weaning), and it has been estimated to affect 40–80% of pigs in the UK and other European countries
. Non-specific colitis is characterized by subacute colitis with mucosal hyperplasia, mononuclear cell infiltration, multifocal mucosal erosions, colonic lesions of increased crypt depth, and poor growth rate
, mainly because of the impaired absorptive functionality and dehydration due to diarrhea. Hyperplasia in colon-associated lymphatic tissue, shining mucosa in colon, and mesenteric lymph node hyperplasia without any related pathogens detected in fecal samples were reported to be considered as the gross pathological signs of NSC
. As mentioned earlier, both SC and NSC induce morphological changes in the colon due to the cascaded inflammatory responses. Knowing the intermediate and possible end-products of these inflammatory mechanisms could be used as possible biomarkers for facilitating the diagnosis of colitis in diarrheal pigs. Calprotectin and lactoferrin are proteins derived from activated neutrophils, and since they are quite stable in feces and could be detected by quantitative ELISA, they are considered as inexpensive and non-invasive biomarkers for colitis in humans
. Calprotectin and lactoferrin have been reported to be closely correlated to the inflammations in patients with IBD when diagnosed by endoscopic measures
. Similarly, in case of colonic inflammation in the pigs, infiltration of neutrophils in the inflamed sites has been reported
for which fecal recovery of calprotectin and lactoferrin could be expected as a promising biomarker of colitis. However, only a few studies have been done on adopting this method for identifying colitis in pigs
.
The exact etiology and epidemiology of NSC is generally poorly understood
. However, several dietary factors seem to be implicated in the development of NSC (
), and these dietary factors may also facilitate pathogenic infections in the large intestine and cause a synergized SC. For instance, NSC was reported to be more prevalent in fast-growing pigs fed high-density diets, i.e., diets with high-metabolizable energy
and the presence of trypsin inhibitors in peas, beans, and soya, and deficiency of vitamin E has also been associated with the occurrence of NSC
. Feeding diets poor in tryptophan, as a precursor of niacin (vitamin B3) biosynthesis, has also been related to the incidence of NSC
. For the biosynthesis of niacin out of tryptophan, the presence of riboflavin, vitamin B
| Vitamin C and E, glutathione, ubiquinol, polyphenols, and β-carotene | Insufficient | Oxidative distress | [33][59][73][74] |
| Essential amino acids | Insufficient | Oxidative stress by reducing antioxidant enzymes, reduced mucin production | [16][17][42][75] |
| Dietary protein | ≥23% | Increased undegraded protein in large intestine and inflammation due to NH4+, reducing gut barrier function and causing NSC | [23][29][42][76] |
| Soluble NSP 2 and RS 3 | Increased | Ameliorating/preventive effect on large intestinal inflammation, increased SCFA 4, and reduced luminal pH | [77][78][79][80][81][82] |
| Pelleted diet | - | Reduced endogenous enzymes in feedstuffs and causing NSC | [68][83] |
2.2.1. Dietary Crude Protein and NSC
Histological examination of colonic tissue of pigs with NSC showed the loss of microvilli and apoptosis of surface epithelial cells, which could be due to the increased concentrations of NH
, indoles, and phenols arising from increased bacterial fermentation of especially undegraded dietary protein
. The increased crypt depth related to NSC was also speculated to be a result of protein fermentation in the colon
. Moreover, increasing the dietary crude protein content from 17 to 23% (
) in five-week-old pigs was shown to be associated with reduced tight junction genes (e.g., Zonula occludens-1 and Occludin) expression in the gut, resulting in increased gut permeability and diarrhea
. In the same study, the incidence of NSC was also related to the increased expression of pro-inflammatory cytokines IL-1β, IFN-γ, and cystic fibrosis transmembrane conductance regulators (CFTR) in the distal colon as a result of increased protein content of the diet
. Upregulation of gene expression of inflammatory cytokines such as IL-1β and IL-6 in the proximal colon of pigs can increase the intestinal epithelial permeability by altering tight junction proteins and disrupting the epithelial integrity
. This reflects the close correlation of these cytokines with the incidence of the colonic inflammation, which could be used as biomarkers for both NSC and SC in pigs. Wu et al.
reported an increased concentration of Cl
and CFTR in the digesta of the terminal colon in piglets fed diets with 23% protein vs. 17%, indicating an increased epithelial permeability and excessive secretion of Cl
due to the increased dietary protein. This can be an indication of the disrupted absorptive functionality of the colon epithelium since luminal accumulation of Cl
ions and CFTR is tightly related to the incidence of diarrhea
, and their quantification in the fecal samples could be used as possible biomarkers for protein-related NSC. An increased level of plasma urea nitrogen in the pigs with diarrhea induced by the increased level of dietary protein was also reported previously
, which could be another indirect indicator of excessive protein fermentation in the colon. In addition, biogenic amines, NH
, indoles, and phenols are byproducts of protein fermentation in the large intestine, and with the recovery assays from the fecal samples, they may also be considered as potential biomarkers of colitis induced by protein fermentation.
References
- Yang, H.; Jiang, W.; Furth, E.E.; Wen, X.; Katz, J.P.; Sellon, R.K.; Silberg, D.G.; Antalis, T.M.; Schweinfest, C.W.; Wu, G.D. Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. Am. J. Physiol. Gastrointest. Liver Physiol. 1998, 275, G1445–G1453.
- Field, M. Intestinal ion transport and the pathophysiology of diarrhea. J. Clin. Investig. 2003, 111, 931–943.
- Surawicz, C.M. Mechanisms of diarrhea. Curr. Gastroenterol. Rep. 2010, 12, 236–241.
- Binder, H.J. Mechanisms of diarrhea in inflammatory bowel diseases. Ann. N. Y. Acad. Sci. 2009, 1165, 285–293.
- Anbazhagan, A.N.; Priyamvada, S.; Alrefai, W.A.; Dudeja, P.K. Pathophysiology of IBD associated diarrhea. Tissue Barriers 2018, 6, e1463897.
- Musch, M.W.; Arvans, D.L.; Wu, G.D.; Chang, E.B. Functional coupling of the downregulated in adenoma Cl-/base exchanger DRA and the apical Na+/H+ exchangers NHE2 and NHE3. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G202–G210.
- Khurana, S.; Ganguly, N.K.; Khullar, M.; Panigrahi, D.; Walia, B.N.S. Studies on the mechanism of Salmonella typhimurium enterotoxin-induced diarrhoea. Biochim. Biophys. Acta Mol. Basis Dis. 1991, 1097, 171–176.
- Kaur, T.; Singh, S.; Gorowara, S.; Ganguly, N.K. Role of enteric nervous system in Shigella dysenteriae type 1 toxin-induced fluid secretion in rabbit ileum. J. Diarrhoeal Dis. Res. 1995, 159–165.
- Moeser, A.J.; Blikslager, A.T. Mechanisms of porcine diarrheal disease. J. Am. Vet. Med. Assoc. 2007, 231, 56–67.
- Vernia, P.; Gnaedinger, A.; Hauck, W.; Breuer, R.I. Organic anions and the diarrhea of inflammatory bowel disease. Dig. Dis. Sci. 1988, 33, 1353–1358.
- Madara, J.L.; Stafford, J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J. Clin. Investig. 1989, 83, 724–727.
- Amasheh, S.; Barmeyer, C.; Koch, C.S.; Tavalali, S.; Mankertz, J.; Epple, H.-J.; Gehring, M.M.; Florian, P.; Kroesen, A.-J.; Zeitz, M.; et al. Cytokine-dependent transcriptional down-regulation of epithelial sodium channel in ulcerative colitis. Gastroenterology 2004, 126, 1711–1720.
- Liu, Y. Fatty acids, inflammation and intestinal health in pigs. J. Anim. Sci. Biotechnol. 2015, 6, 41.
- Schulzke, J.D.; Ploeger, S.; Amasheh, M.; Fromm, A.; Zeissig, S.; Troeger, H.; Richter, J.; Bojarski, C.; Schumann, M.; Fromm, M. Epithelial tight junctions in intestinal inflammation. Ann. N. Y. Acad. Sci. 2009, 1165, 294–300.
- Caruso, R.; Lo, B.C.; Núñez, G. Host–microbiota interactions in inflammatory bowel disease. Nat. Rev. Immunol. 2020, 20, 411–426.
- Faure, M.; Mettraux, C.; Moennoz, D.; Godin, J.-P.; Vuichoud, J.; Rochat, F.; Breuillé, D.; Obled, C.; Corthésy-Theulaz, I. Specific amino acids increase mucin synthesis and microbiota in dextran sulfate sodium–treated rats. J. Nutr. 2006, 136, 1558–1564.
- Puiman, P.J.; Jensen, M.; Stoll, B.; Renes, I.B.; de Bruijn, A.C.J.M.; Dorst, K.; Schierbeek, H.; Schmidt, M.; Boehm, G.; Burrin, D.G.; et al. Intestinal Threonine Utilization for Protein and Mucin Synthesis Is Decreased in Formula-Fed Preterm Pigs. J. Nutr. 2011, 141, 1306–1311.
- Van der Sluis, M.; De Koning, B.A.; De Bruijn, A.C.; Velcich, A.; Meijerink, J.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenteritis 2006, 131, 117–129.
- Specian, R.D.; Oliver, M.G. Functional biology of intestinal goblet cells. Am. J. Physiol. Cell Physiol. 1991, 260, C183–C193.
- Fuss, I.J.; Strober, W. Chapter 81—Ulcerative Colitis. In Mucosal Immunology, 4th ed.; Mestecky, J., Strober, W., Russell, M.W., Kelsall, B.L., Cheroutre, H., Lambrecht, B.N., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 1573–1612.
- Martin, C.R.; Walker, W.A. Chapter 70—Innate and Mucosal Immunity in the Developing Gastrointestinal Tract: Relationship to Early and Later Disease. In Avery’s Diseases of the Newborn, 9th ed.; Gleason, C.A., Devaskar, S.U., Eds.; W.B. Saunders: Philadelpia, PA, USA, 2012; pp. 994–1006.
- Bengtsson, R.J.; MacIntyre, N.; Guthrie, J.; Wilson, A.D.; Finlayson, H.; Matika, O.; Pong-Wong, R.; Smith, S.H.; Archibald, A.L.; Ait-Ali, T. Lawsonia intracellularis infection of intestinal crypt cells is associated with specific depletion of secreted MUC2 in goblet cells. Vet. Immunol. Immunopathol. 2015, 168, 61–67.
- Wu, Y.; Jiang, Z.; Zheng, C.; Wang, L.; Zhu, C.; Yang, X.; Wen, X.; Ma, X. Effects of protein sources and levels in antibiotic-free diets on diarrhea, intestinal morphology, and expression of tight junctions in weaned piglets. Anim. Nutr. 2015, 1, 170–176.
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246.
- Stege, H.; Jensen, T.K.; Møller, K.; Baekbo, P.; Jorsal, S. Risk factors for intestinal pathogens in Danish finishing pig herds. Prev. Vet. Med. 2001, 50, 153–164.
- Pedersen, K.S.; Johansen, M.; Angen, O.; Jorsal, S.E.; Nielsen, J.P.; Jensen, T.K.; Guedes, R.; Ståhl, M.; Bækbo, P. Herd diagnosis of low pathogen diarrhoea in growing pigs—A pilot study. Ir. Vet. J. 2014, 67, 24.
- Thomson, J.; Smith, W.J.; Fowler, V.R.; Edwards, S.; Hazzledine, M. Non-specific colitis in pigs: Defining the condition. In Proceedings of the 17th International Pig Veterinary Society Congress, Ames, IA, USA, 2–5 June 2002.
- Constable, P.; Hinchcliff, K.; Done, S.; Grünberg, W. 7—Diseases of the Alimentary Tract: Nonruminant. In Veterinary Medicine, 11st ed.; Constable, P.D., Hinchcliff, K.W., Done, S.H., Grünberg, W., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2017; pp. 175–435.
- Luise, D.; Lauridsen, C.; Bosi, P.; Trevisi, P. Methodology and application of Escherichia coli F4 and F18 encoding infection models in post-weaning pigs. J. Anim. Sci. Biotechnol. 2019, 10, 53.
- Burrough, E.R. Swine Dysentery: Etiopathogenesis and Diagnosis of a Reemerging Disease. Vet. Pathol. 2016, 54, 22–31.
- Zachary, J.F. Chapter 4—Mechanisms of Microbial Infections1. In Pathologic Basis of Veterinary Disease, 6th ed.; Zachary, J.F., Ed.; Mosby: Maryland Heights, MO, USA, 2017; pp. 132–241.e1.
- Hampson, D.J.; Burrough, E.R. Swine Dysentery and Brachyspiral Colitis. In Diseases of Swine; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 951–970.
- Carr, J.; Chen, S.-P.; Connor, J.F.; Kirkwood, R.; Segalés, J. Pig Health; CRC Press: Boca Raton, FL, USA, 2018.
- Stanton, T.B. The Genus Brachyspira. Prokar 2006, 7, 330–356.
- Jensen, T.; Boye, M.; Møller, K. Extensive intestinal spirochaetosis in pigs challenged with Brachyspira pilosicoli. J. Med. Microbiol. 2004, 53, 309–312.
- Trott, D.J.; Huxtable, C.R.; Hampson, D.J. Experimental infection of newly weaned pigs with human and porcine strains of Serpulina pilosicoli. Infect. Immun. 1996, 64, 4648–4654.
- Lawhorn, D.B. Diarrheal disease in show swine. Tex. FARMER Collect. 2007, 3, 439.
- Kringel, H.; Iburg, T.; Dawson, H.; Aasted, B.; Roepstorff, A. A time course study of immunological responses in Trichuris suis infected pigs demonstrates induction of a local type 2 response associated with worm burden. Int. J. Parasitol. 2006, 36, 915–924.
- Pittman, J.S.; Shepherd, G.; Thacker, B.J.; Myers, G.H. Trichuris suis in finishing pigs: Case report and review. J. Swine Health Prod. 2010, 18, 306–313.
- Roepstorff, A.; Mejer, H.; Nejsum, P.; Thamsborg, S.M. Helminth parasites in pigs: New challenges in pig production and current research highlights. Vet. Parasitol. 2011, 180, 72–81.
- Leroux, L.-P.; Nasr, M.; Valanparambil, R.; Tam, M.; Rosa, B.A.; Siciliani, E.; Hill, D.E.; Zarlenga, D.S.; Jaramillo, M.; Weinstock, J.V.; et al. Analysis of the Trichuris suis excretory/secretory proteins as a function of life cycle stage and their immunomodulatory properties. Sci. Rep. 2018, 8, 15921.
- Thomson, J. Feed-associated colitis of growing pigs and its interaction with enteric infections. Acta Sci. Vet. 2009, 37, s1–s9.
- Gelberg, H.B. Chapter 7—Alimentary System and the Peritoneum, Omentum, Mesentery, and Peritoneal Cavity1. In Pathologic Basis of Veterinary Disease, 6th ed.; Zachary, J.F., Ed.; Mosby: Maryland Heights, MO, USA, 2017; pp. 324–411.e1.
- Argenzio, R.A.; Whipp, S.C.; Glock, R.D. Pathophysiology of Swine Dysentery: Colonic Transport and Permeability Studies. J. Infect. Dis. 1980, 142, 676–684.
- Stege, H.; Jensen, T.K.; Møller, K.; Bækbo, P.; Jorsal, S.E. Prevalence of intestinal pathogens in Danish finishing pig herds. Prev. Vet. Med. 2000, 46, 279–292.
- Quintana-Hayashi, M.P.; Mahu, M.; De Pauw, N.; Boyen, F.; Pasmans, F.; Martel, A.; Premaratne, P.; Fernandez, H.R.; Teymournejad, O.; Maele, L.V. The levels of Brachyspira hyodysenteriae binding to porcine colonic mucins differ between individuals, and binding is increased to mucins from infected pigs with de novo MUC5AC synthesis. Infect. Immun. 2015, 83, 1610–1619.
- Jin, L.; Reynolds, L.P.; Redmer, D.A.; Caton, J.S.; Crenshaw, J.D. Effects of dietary fiber on intestinal growth, cell proliferation, and morphology in growing pigs2. J. Anim. Sci. 1994, 72, 2270–2278.
- Pedersen, K.S.; Kristensen, C.S.; Nielsen, J.P. Demonstration of non-specific colitis and increased crypt depth in colon of weaned pigs with diarrhea. Vet. Q. 2012, 32, 45–49.
- Casas, V.; Vadillo, S.; San Juan, C.; Carrascal, M.; Abian, J. The Exposed Proteomes of Brachyspira hyodysenteriae and B. pilosicoli. Front. Microbiol. 2016, 7, 1103.
- Wyns, H.; Plessers, E.; De Backer, P.; Meyer, E.; Croubels, S. In vivo porcine lipopolysaccharide inflammation models to study immunomodulation of drugs. Vet. Immunol. Immunopathol. 2015, 166, 58–69.
- Gessner, D.K.; Fiesel, A.; Most, E.; Dinges, J.; Wen, G.; Ringseis, R.; Eder, K. Supplementation of a grape seed and grape marc meal extract decreases activities of the oxidative stress-responsive transcription factors NF-κB and Nrf2 in the duodenal mucosa of pigs. Acta Vet. Scand. 2013, 55, 18.
- Lallès, J.-P.; Fagerhol, M.K. Faecal calprotectin: A non invasive marker of inflammation in pigs. ISAH 2005, 1, 405–408.
- Lamb, C.A.; Mansfield, J.C. Measurement of faecal calprotectin and lactoferrin in inflammatory bowel disease. Frontline Gastroenterol. 2011, 2, 13.
- Yamamoto, T.; Shiraki, M.; Bamba, T.; Umegae, S.; Matsumoto, K. Faecal calprotectin and lactoferrin as markers for monitoring disease activity and predicting clinical recurrence in patients with Crohn’s disease after ileocolonic resection: A prospective pilot study. United Eur. Gastroenterol. J. 2013, 1, 368–374.
- Bogere, P.; Choi, Y.J.; Heo, J. Optimization of Fecal Calprotectin Assay for Pig Samples. J. Agric. Life Sci. 2019, 53, 93–104.
- Jobin, C.; Hellerbrand, C.; Licato, L.L.; Brenner, D.A.; Sartor, R.B. Mediation by NF-kappa B of cytokine induced expression of intercellular adhesion molecule 1 (ICAM-1) in an intestinal epithelial cell line, a process blocked by proteasome inhibitors. Gut 1998, 42, 779–787.
- Rogler, G.; Brand, K.; Vogl, D.; Page, S.; Hofmeister, R.; Andus, T.; Knuechel, R.; Baeuerle, P.A.; Schölmerich, J.; Gross, V. Nuclear factor κB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology 1998, 115, 357–369.
- Ungaro, F.; Rubbino, F.; Danese, S.; D’Alessio, S. Actors and Factors in the Resolution of Intestinal Inflammation: Lipid Mediators as a New Approach to Therapy in Inflammatory Bowel Diseases. Front. Immunol. 2017, 8, 1331.
- Pistol, G.C.; Marin, D.E.; Rotar, M.C.; Ropota, M.; Taranu, I. Bioactive compounds from dietary whole grape seed meal improved colonic inflammation via inhibition of MAPKs and NF-κB signaling in pigs with DSS induced colitis. J. Funct. Foods 2020, 66, 103708.
- Tan, C.; Wei, H.; Sun, H.; Ao, J.; Long, G.; Jiang, S.; Peng, J. Effects of dietary supplementation of oregano essential oil to sows on oxidative stress status, lactation feed intake of sows, and piglet performance. BioMed Res. Int. 2015, 2015, 525218.
- Schuh, S.; Muller, L.K.; Campos, L.P.; Moresco, R.N.; Baldissera, M.D.; de Oliveira, S.C.; Campigotto, G.; Da Silva, A.S.; Paiano, D. Effect of supplementation of newborn piglets with spray dry blood plasma on weight gain and serum biochemical variables. Comp. Clin. Path. 2016, 25, 1029–1033.
- Rubio, C.P.; Mainau, E.; Cerón, J.J.; Contreras-Aguilar, M.D.; Martínez-Subiela, S.; Navarro, E.; Tecles, F.; Manteca, X.; Escribano, D. Biomarkers of oxidative stress in saliva in pigs: Analytical validation and changes in lactation. BMC Vet. Res. 2019, 15, 144.
- Pothoulakis, C.; Castagliuolo, I.; LaMont, J.T. Nerves and intestinal mast cells modulate responses to enterotoxins. Physics 1998, 13, 58–63.
- Argenzio, R.A. Glucose-stimulated fluid absorption in the pig small intestine during the early stage of swine dysentery. Am. J. Vet. Res. 1980, 41, 2000–2006.
- Laber, K.E.; Whary, M.T.; Bingel, S.A.; Goodrich, J.A.; Smith, A.C.; Swindle, M.M. Chapter 15—Biology and Diseases of Swine. In Laboratory Animal Medicine, 2nd ed.; Fox, J.G., Anderson, L.C., Loew, F.M., Quimby, F.W., Eds.; Academic Press: Burlington, NJ, USA, 2002; pp. 615–673.
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259.
- Hill, D.E.; Romanowski, R.D.; Urban, J.F. A Trichuris specific diagnostic antigen from culture fluids of Trichuris suis adult worms. Vet. Parasitol. 1997, 68, 91–102.
- Chase-Topping, M.E.; Gunn, G.; Strachan, W.D.; Edwards, S.A.; Smith, W.J.; Hillman, K.; Stefopoulou, S.N.; Thomson, J.R. Epidemiology of porcine non-specific colitis on Scottish farms. Vet. J. 2007, 173, 353–360.
- Taylor-Pickard, J.A.; Stevenson, Z.; Glebocka, K. Formula for the Future: Nutrition or Pathology? Elevating Performance and Health in Pigs and Poultry; Wageningen Academic Pub: Wageningen, The Netherlands, 2008.
- Luecke, R.; McMillen, W.; Thorp, F., Jr.; Tull, C. The relationship of nicotinic acid, tryptophane and protein in the nutrition of the pig. J. Nutr. 1947, 33, 251–261.
- Si, Y.; Zhang, Y.; Zhao, J.; Guo, S.; Zhai, L.; Yao, S.; Sang, H.; Yang, N.; Song, G.; Gu, J.; et al. Niacin Inhibits Vascular Inflammation via Downregulating Nuclear Transcription Factor-B Signaling Pathway. Mediat. Inflamm. 2014, 2014, 263786.
- Salem, H.; Wadie, W. Effect of Niacin on Inflammation and Angiogenesis in a Murine Model of Ulcerative Colitis. Sci. Rep. 2017, 7, 7139.
- Thérond, P.; Bonnefont-Rousselot, D.; Davit-Spraul, A.; Conti, M.; Legrand, A. Biomarkers of oxidative stress: An analytical approach. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 373–384.
- Lowe, F. Biomarkers of Oxidative Stress. Syst. Biol. Free Radic. Antioxid. 2014, 22.
- Toledo, J.B.; Furlan, A.C.; Pozza, P.C.; Piano, L.M.; Carvalho, P.L.O.; Peñuela-Sierra, L.M.; Huepa, L.M.D. Effect of the reduction of the crude protein content of diets supplemented with essential amino acids on the performance of piglets weighing 6–15kg. Livest. Sci. 2014, 168, 94–101.
- Nyachoti, C.M.; Omogbenigun, F.O.; Rademacher, M.; Blank, G. Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets. J. Anim. Sci. 2006, 84, 125–134.
- Molist, F.; van Oostrum, M.; Pérez, J.F.; Mateos, G.G.; Nyachoti, C.M.; van der Aar, P.J. Relevance of functional properties of dietary fibre in diets for weanling pigs. Anim. Feed Sci. Technol. 2014, 189, 1–10.
- Jha, R.; Fouhse, J.M.; Tiwari, U.P.; Li, L.; Willing, B.P. Dietary Fiber and Intestinal Health of Monogastric Animals. Front. Vet. Sci. 2019, 6, 48.
- Regassa, A.; Nyachoti, C.M. Application of resistant starch in swine and poultry diets with particular reference to gut health and function. Anim. Nutr. 2018, 4, 305–310.
- Souza da Silva, C.; van den Borne, J.J.G.C.; Gerrits, W.J.J.; Kemp, B.; Bolhuis, J.E. Effects of dietary fibers with different physicochemical properties on feeding motivation in adult female pigs. Physiol. Behav. 2012, 107, 218–230.
- Haenen, D.; Zhang, J.; Souza da Silva, C.; Bosch, G.; van der Meer, I.M.; van Arkel, J.; van den Borne, J.J.G.C.; Pérez Gutiérrez, O.; Smidt, H.; Kemp, B.; et al. A Diet High in Resistant Starch Modulates Microbiota Composition, SCFA Concentrations, and Gene Expression in Pig Intestine. J. Nutr. 2013, 143, 274–283.
- Yang, X.; Darko, K.O.; Huang, Y.; He, C.; Yang, H.; He, S.; Li, J.; Li, J.; Hocher, B.; Yin, Y. Resistant Starch Regulates Gut Microbiota: Structure, Biochemistry and Cell Signalling. Cell. Physiol. Biochem. 2017, 42, 306–318.
- Thomson, J.R.; Smith, W.J.; Murray, B.P. Investigations into field cases of porcine colitis with particular reference to infection with Serpulina pilosicoli. Vet. Rec. 1998, 142, 235.
- Al-Sadi, R.; Boivin, M.; Ma, T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. J. Virt. Lib. 2009, 14, 2765.
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Gabler, N.K. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS ONE 2013, 8, e70215.
- Berger, A.L.; Ikuma, M.; Welsh, M.J. Normal gating of CFTR requires ATP binding to both nucleotide-binding domains and hydrolysis at the second nucleotide-binding domain. Proc. Natl. Acad. Sci. USA. 2005, 102, 455–460.