You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Ana Maia.
Cancer-associated fibroblasts are important players of the tumour microenvironment. They influence numerous processes during tumour development and progression, including the response of cancer cells to treatment. As a consequence, this cell type has emerged has a prominent target in anti-cancer therapy.
- cancer associated fibroblast (CAF)
- tumour microenvironment (TME)
- cell communication
- signalling
- therapy resistance
1. Introduction
The observation that tumour cells do not act as an isolated entity but, instead, interact with other cells in the human body was proposed in the mid-nineteen century by Rudolph Virchow who first established a link between inflammation and tumour development [1]. A further suggestion of the importance of the interactions of cancer cells and their microenvironment arose a few years later when Stephen Paget observed that disseminated tumour cells preferentially colonise certain organs and coined the famous ‘Seed and soil hypothesis’ [2]. Nowadays, a huge body of evidence has been obtained for the role of the TME in determining numerous aspects of tumour development, progression, metastasis development and therapeutic response [3]. The term TME (also named stroma) comprises a broad panoply, including all the components that surround the cancer cells, namely immune cells, vasculature, extracellular matrix (ECM) and fibroblasts, as well as their interactions [4].
The TME closely interacts with the malignant cells and can have either tumour restrictive or promoting effects. Moreover, these elements are not static and, as the tumour develops, the tumour cells modulate their environment and often highjack it to promote their growth and progression. An important and dominant component of the tumour stroma with broadly described pro-tumorigenic capacity is the cancer-associated fibroblast (CAF) [5]. CAFs are phenotypically different from normal quiescent fibroblasts and have been described to resemble fibroblasts in the context of wound healing due to their enhanced secretion of ECM components as well as other soluble factors such as cytokines and chemokines [6,7][6][7]. This is now known to be true for a subset of fibroblasts present in the tumour microenvironment, which can be identified based on their contractile characteristics and expression of certain proteins such as alpha smooth muscle actin (αSMA) [8,9][8][9]. However, recent advances in molecular technologies such as single-cell sequencing [10] have made the further dissection of stromal fibroblasts possible and has shown that this is not the whole story. Nevertheless, challenges remain in the characterization of fibroblasts and, consequently, their therapeutic targeting.
2. Fibroblasts
Fibroblasts were first described in the 19th century by Virchow [11]. These mesenchymal-derived cells with a spindle-like appearance are the main cellular component of connective tissue and are responsible for the synthesis of the majority of the constituents of the fibrillar ECM, including collagens, elastin and fibronectin, which allows them to regulate the morphology of tissues [12]. In wound-healing, the role of these cells is very well established and the morphological and phenotypic changes that fibroblasts undergo have been widely described [13,14,15][13][14][15]. Even though the first description of these cells was almost two centuries ago, fibroblasts are often still identified based on their morphology, tissue location and lack of epithelial markers. The absence of specific markers that would allow the identification of this cell type has remained one of the biggest challenges in the study of fibroblasts [16]. Despite the lack of a universal marker, fibroblasts have thus far been identified by using a combination of commonly expressed mesenchymal markers and other proteins associated with certain activation states. The markers that are common, but not exclusive, to fibroblasts, regardless of their activation state, include vimentin [17], fibroblast-specific protein 1 (FSP-1, also known as S100A4) [18,19][18][19] and platelet-derived growth factor receptor α (PDGFRα) [20]. Several other markers have been shown to detect specific activation states of fibroblasts and, consequently, define subpopulations of fibroblasts. Among these, the most universally used are αSMA and fibroblast-activation protein α (FAPα) [21,22][21][22]. In addition to these, several markers specific for certain tissues have been identified. This high level of heterogeneity further complicates the connection between findings from one study to another, since uniformity in analysing this cell type is then missing. Novel advances in single-cell omics can overcome this major drawback and have already proven invaluable for advancing our knowledge in fibroblast biology.
The subpopulations of fibroblasts in healthy tissues are a reflection of different transcriptomic and epigenetic profiles that are dictated by the embryonic origin and architecture of the tissue as well as their microenvironment [23,24][23][24]. Using a variety of in vivo models, several studies aimed at dissecting fibroblast heterogeneity have identified numerous distinct subpopulations of fibroblasts in several tissues and diseases [24]. In humans, due to the higher complexity of sampling healthy tissues, studies have thus far extensively focused on skin fibroblasts, but also include other tissues. There, comparable to what was found in mouse models, several functionally distinct subpopulations have been shown to exist [25,26][25][26]. If fibroblasts in healthy tissues are already characterised by a high degree of heterogeneity, the picture in tumours is even more complex, not only due to possibly distinct origins [23], but also as a result of the dynamics and selective pressure that characterises tumour evolution [27]. Fibroblasts in the TME are exposed to a variety of factors, such as cytokines, chemokines and growth factors, whose composition can reprogram and dictate the phenotype of these cells. Several molecules have been described to activate and promote the pro-tumorigenic states of fibroblasts, including TGF-β, PDGF, FGF2, HGF, IL-1 and IL-6, among others [9]. In addition to reprogramming by secreted factors, fibroblasts have been shown to be activated by different stress sources, such as ROS and damaging therapy, and well as by alterations in the ECM [16].
A clear evidence of functional heterogeneity in fibroblasts present in the TME arose with the first trials to target this cell type in vivo. In pancreatic cancer, the depletion of αSMA-expressing fibroblasts from the TME accelerated tumour growth and resulted in an immunosuppressive TME [28,29][28][29]. Interestingly, the opposite effect was observed when CAFs expressing FAP were therapeutically targeted, with this resulting in an impairment of tumour growth [30,31][30][31]. As observed in a study by Özdemir and colleagues, this was also a result of alterations in the immune milieu of the tumours. Contrary to what happened with αSMA-expressing CAFs, the depletion of FAP-positive CAFs enhanced adaptive immunity and reduced immunosuppression in the TME [30,31,32][30][31][32]. This clearly illustrates that prior to any attempt to target these cells in the clinic, an improvement in our understanding in the biology of fibroblasts is essential. With this in mind, numerous groups have attempted to resolve this challenging problem and we will describe the main findings below. Although we have seen an explosion of studies whose goal is to unravel both the molecular and functional heterogeneity of CAFs, most of these have focused on investigating the composition of fibroblasts at different stages of tumour development, still leaving the picture incomplete. The impact of therapies in subpopulations of fibroblasts has been thus far largely overlooked and studies that investigate the evolution of fibroblasts alongside with other cell types, such as tumour and other TME cells, are absent. This could provide important insights in the biology of fibroblasts in therapy response and provide essential knowledge for the rational targeting of this cell type or of its interactions with tumour cells.
2.1. Fibroblast Heterogeneity
Prior to the development of single-cell sequencing, fibroblasts were often characterised based on the expression of pre-defined markers using flow cytometry analysis or immunohistochemistry. Despite the power of these techniques to provide single-cell and spatial resolution, an unbiased approach, in which the full transcriptome of fibroblasts was analysed without the pre-selection of certain markers, was missing. Bulk RNA-sequencing of fibroblast-enriched fractions based on negative selection provided a broad overview of gene expression in these cells but lacked the single-cell resolution. The development and establishment of single-cell RNA sequencing (scRNA-seq) provided the cornerstone for the study of tumour heterogeneity, including fibroblast heterogeneity. The development of microfluidic platforms that allowed a dramatic improvement in the power of analysis by increasing the number of sequenced cells [33], as well as the more recently proposed method of sequential indexing, which promises to exponentially increase the number of cells that can be sequenced at the single-cell resolution [34], are crucial developments to allow us to dissect the biology of fibroblasts in the context of cancer.
Based on the analysis of different tumour entities and using single-cell technologies, several fibroblast subpopulations have been identified and their roles in different aspects of the tumour start to be unravelled. The most well studied entities include breast [35,36,37][35][36][37] and pancreatic carcinomas [38[38][39][40][41][42],39,40,41,42], although studies in other tumour models are starting to appear [43,44,45,46,47][43][44][45][46][47]. Despite the context-dependent variability, two recurrent subpopulations that have now been identified in several tumour entities are inflammatory CAFs (iCAFs) and myofibroblasts (myCAFs). Very briefly, these subpopulations are functionally characterised by the secretion of inflammatory cytokines and cytoskeleton remodelling, respectively [38,40,46,48,49,50][38][40][46][48][49][50]. In addition to different transcriptional profiles, these subpopulations were shown to be located in distinct areas of the tumour, with myCAFs being found in closer proximity to malignant cells and iCAFs localising distally [38]. More importantly, these subpopulations have been shown to be functionally discrete and mutually exclusive [38,51][38][51]. Using single-cell sequencing, Öhlund et al. [38] first described these subpopulations in a model of pancreatic cancer. Their findings were further confirmed by other studies, where the transcriptional program of myCAFs was shown to be driven by TGF-β signalling and these cells were characterised by the expression of high levels of αSMA. On the other hand, the activation of the NF-κB pathway driven by IL-1β led to the acquisition of an iCAF phenotype. These cells were characterised by the secretion numerous inflammatory cytokines, including IL-6, CXCL1, CXCL12, among others [38,39,45][38][39][45]. These two subpopulations also seem to be regulated by sonic hedgehog (Shh) signalling, whose activation supported the myCAF phenotype [52]. Interestingly, iCAFs were shown to exclusively express HAS1 and HAS2, which are responsible for the synthesis of hyaluronan, a component of the ECM, which has been shown to work as a barrier in the treatment of pancreatic cancer [39]. In breast cancer, our group has shown that a subset of fibroblasts in the luminal subtype is characterised by the expression of NRG1, whose expression correlated with HAS2 expression [53]. Moreover, Biffi et al. have shown that the two fibroblast states, iCAF and myCAF, seem to be mutually exclusively, since exposure to TGF-β resulted in the downregulation of the IL-1β receptor (IL-1R1) and, consequently, led to the differentiation of fibroblasts into myCAFs [51].
The observation that these two fibroblast populations are recurrently found in the microenvironment of several tumour entities could arise from the fact that these are numerically dominant subpopulations. Nevertheless, this does not invalidate the existence of other subpopulations. In fact, several other subpopulations have been shown to co-exist, although these were more variable and different compositions have been identified according to the tumour entity studied. Moreover, it could be that these dominant subpopulations can still be further divided into further distinct functional clusters. In a recent study, Friedman et al. show three different clusters of inflammatory CAFs, each one being characterised by the expression of IL-6, CXCL1 and CXCL12. However, a real functional distinction between the three clusters is not shown in this study [36]. Furthermore, using flow cytometry, Costa et al. showed that fibroblasts in breast cancer could be divided into four populations (CAF-S1 to S4), according to the expression of commonly used markers such as FAPα, αSMA, FSP1, PDGFRβ and Caveolin, demonstrating an immunosuppressive role of CAF-S1 in the TME [37]. By analysing almost 20,000 cells from this CAF subpopulation using single-cell sequencing, Kieffer et al. could divide CAF-S1 into eight distinct clusters. They further show that fibroblasts from cluster zero and three were characterised by TGF-β signalling and high αSMA expression and that these cross-talked with T-cells in the TME, driving a regulatory phenotype in the T-cells (Treg) [54], which explains the immunosuppressive role initially proposed by the same group [37]. As described above, the targeting of all αSMA-positive cells and the depletion of these from the TME by Özdemir et al. led to the outgrowth of pancreatic tumours [28,29][28][29]. Although the study from Kieffer et al. focused on breast cancer, their findings clearly raise the question of whether the targeting of specific clusters of αSMA-positive fibroblasts could indeed be beneficial for patients to elicit an efficient T-cell mediated immunity against malignant cells. This highlights the importance of the advances seen in single-cell omics that allow us to improve the power of single-cell analyses and that will help us further dissect the complexity of fibroblasts in cancer.
Yet another interesting subpopulation that has also been recently identified in pancreatic cancer is termed an antigen-presenting CAF (apCAF). These are characterised by the expression of genes associated with MHC-class II antigen presentation [39], although they lack co-stimulatory ligands necessary to activate immune cells. Instead, MHC-II signalling in this subpopulation of fibroblasts seems to drive the expansion of regulatory T cells and, in this way, promote an immunosuppressive microenvironment [39]. Despite all these novel findings, further functional characterisation and validation of the importance of these different subpopulations in patients is still needed. Another layer of complexity arises when these subpopulations are analysed in the context of time, with studies showing alterations in the composition of these subpopulations between early and late tumours [36,40,42,45][36][40][42][45].
In the next section, we will describe the current knowledge on how fibroblasts affect the response of tumour cells to anti-cancer therapies and try to correlate the subpopulations of fibroblasts described above with these events. We mostly focus on interactions that result in a negative impact on therapy response and can, ultimately, result in therapeutic failure. Nevertheless, it is important to mention that not all interactions between CAFs and cancer cells drive tumour progression or resistance to therapy. Numerous studies have shown that CAFs can have anti-tumorigenic functions in the TME [55], strengthening the view that further dissection and understanding of the phenotypes and functions of fibroblasts is essential prior to their therapeutic targeting.
2.2. Fibroblast and Therapy Response
Cancer cells can exploit a variety of mechanisms to escape therapy-induced cell death or irreversible cell cycle arrest. These can be categorised into (1) drug-dependent mechanisms, (2) target-dependent mechanisms or (3) drug- and target-independent mechanisms. Very briefly, drug-dependent mechanisms include any alteration that interferes with the availability of the drug. Mutations in the target protein that interfere with the drug activity or alterations in signalling pathways that overcome target inhibition are some examples of target-dependent mechanisms. Finally, the last category includes the acquisition of new aberrations that switch the growth dependency of tumour cells to a different protein and/or pathway as well as alterations in apoptotic pathways [56]. The TME dictates the way cancer cells respond to therapy. Several studies investigating mechanisms of resistance nicely showed that in vivo resistant cancer cells lose their resistant phenotype when they are removed from their microenvironment and are grown alone in vitro [57,58][57][58]. Fibroblasts can influence the vast majority of the mechanisms mentioned above by either (1) supporting specific subpopulations of cancer cells, such as stem-like cancer cells [59,60[59][60][61][62][63],61,62,63], (2) rewiring the signalling networks in cancer cells [64,65,66,67][64][65][66][67] and (3) modulating the tumour microenvironment, namely immune cells [68]. Interestingly, spatial analysis has revealed that the close proximity of malignant cells to stromal fibroblasts is associated with an increased cycling capacity of cancer cells after therapy, hinting to a protective role of fibroblasts [69].
A large number of studies have shown that, through a variety of mechanisms, fibroblasts promote resistance to therapy due to their interplay with cancer stem cells (CSCs). This is especially true for the current standard of care therapies that often depend on the proliferative state of cancer cells, such as radiotherapy and chemotherapy. This is an indirect effect, since fibroblasts promote the expansion of the subset of cancer cells with stem-like properties that have been extensively described to be intrinsically more resistant to numerous therapies, but do not directly modulate the way cancer cells respond to the agent they are exposed to. A more direct mechanism through which fibroblasts can modulate the response of cancer cells to therapy is by activating alternative signalling mechanisms, such as MAPK and PI3K signalling, that provide pro-survival signals and, thus, prevent cancer cells from undergoing apoptosis. Interestingly, co-culture of fibroblasts with healthy cells drove a similar rewiring of the same signalling networks as to when oncogenic mutations, such as the classical G12D mutation in KRAS, were introduced in these healthy cells [70]. Moreover, several of the above-described therapies, including chemotherapy, depend on a functional immune system for effective tumour elimination. This effect is also known as immunogenic cell death (ICD), in which the cytotoxic agents-induced cell death of cancer cells leads to the recruitment of immune cells that enhance the efficacy of these drugs [71,72,73,74,75][71][72][73][74][75]. By modulating the immune landscape in the tumour microenvironment [68], fibroblasts can also affect the response of cancer cells to a multitude of agents including chemotherapies. As expected, the influence of fibroblasts on the immune subpopulations and immune infiltrate in the tumour is of major importance in determining the outcome of cancer cells to several immunotherapies. These processes are shown in Figure 1 and will be described in detail in the sections below.
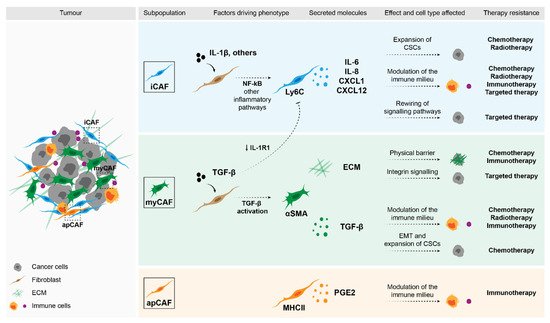

Figure 1. Impact of fibroblasts in therapy response. Stromal fibroblasts can drive therapy resistance through a variety of mechanisms. Factors that were shown to be secreted by specific subpopulations of CAFs can directly affect malignant cells and drive the expansion of CSCs (e.g., cytokines such as IL-6, IL-8 and CXCL1), or activate pro-survival pathways (e.g., ECM through integrin signalling). Moreover, these secreted molecules can modulate the immune populations in the TME and in this way, determine therapy response.
2.2.1. Cytotoxic Agents—Chemotherapy and Radiotherapy
An important way through which fibroblasts modulate the response of tumours to cytotoxic agents, such as chemotherapy and radiotherapy, is via their interaction with CSCs. These cells are at the top of the tumour hierarchy since, in addition to their self-renewal capacity, they are also able to differentiate into committed tumour cells and, consequently, repopulate the whole tumour [76]. CSCs have been shown to be intrinsically more resistant to these agents, not only due to an enhanced DNA damage repair mechanisms or a higher expression of ATP-binding cassette (ABC) transporters [77], but also due to their cycling state. CSCs are often in a dormant, non-proliferative state [78] that makes them relatively refractory to treatments that target an active proliferative state in cells. A mechanism through which fibroblasts have been shown to drive the expansion of the CSCs fraction in tumours was described by Su and colleagues [60]. The authors showed that a subpopulation of fibroblasts characterised by the expression of two markers, CD10 and GPR77, promoted chemoresistance by driving the survival of CSCs in both breast and lung cancer models. Interestingly, they showed that this subpopulation of fibroblasts is present in the tumours prior to any treatment, and that it becomes enriched upon therapy. CD10+GPR77+ fibroblasts were characterised by the activation of the NF-κB pathway, and their secretion of IL-6 as well as IL-8 was essential to drive resistance to chemotherapy. Based on more recent knowledge on the different subsets of fibroblasts, it could be hypothesised that these GPR77- and CD10-positive fibroblasts form a subcluster of the iCAFs. Kanzaki [79] has shown that, in breast cancer, the expression of CD10 and GPR77 is restricted to one of the clusters identified in a study from Bartoschek and colleagues [35]. Unfortunately, in this early study, no inflammatory CAF cluster was defined and the parallel is thus hard to establish. Nevertheless, the study from Su et al. further highlights the importance of uncovering fibroblast heterogeneity in the context of cancer. Moreover, it supports the significance of regarding the fibroblast dynamics during therapy. Several other studies have described mechanisms through which fibroblasts can support and expand tumour-initiating cells. Boelens et al. uncovered a paracrine crosstalk between cancer cells and stromal fibroblasts in which RIG-I and NOTCH signalling cooperate to drive the expansion of therapy resistant CSCs [59]. Briefly, they showed that exosomes secreted by stromal fibroblasts activate the anti-viral machinery in cancer cells via STAT1, which, in turn, drives the expression of interferon-stimulated genes (ISGs). This signature had previously been identified as a gene signature for radiotherapy and chemotherapy resistance [80,81][80][81] and was termed as interferon-related DNA damage resistance signature (IRDS). By facilitating the expression of NOTCH target genes in a STAT1-dependent manner, the stromal interaction with cancer cells drove the expansion of CSCs and, consequently, resistance to chemotherapy. Further studies have implicated JAK-STAT and NF-κB signalling in CAFs in the support of tumour-initiating cells. In pancreatic cancer, Chan et al. demonstrated that the secretion of ELR+ chemokines, CXCL1, CXCL2, CXCL5 and CXCL6 by CAFs bind and activate the CXCR2 downstream signalling in cancer cells, driving the expansion of CSCs [82]. Other pathways that modulate stem-like features in cancer cells have also been described to be activated by fibroblasts, namely Hedgehog [61] and Wnt signalling [83,84][83][84].
Several of the mechanisms described above seem to be mediated by iCAFs. Nevertheless, the myCAFs and ECM they synthesise can also strongly affect the way tumours respond to therapy. For example, a desmoplastic tumour can create a physical barrier that will prevent the exposure of cancer cells to the drug [85,86][85][86]. Moreover, the activation of integrin signalling in cancer cells as a result of a dense ECM, can promote the survival of malignant cells [87,88][87][88]. Modulation of the ECM by fibroblast-derived Anexin A6-loaded extracellular vesicles (EVs) also resulted in integrinβ1-FAK-YAP activation and drove chemoresistance in gastric cancer [89]. The secretion of immunosuppressive factors such as TGF-β by myCAFs can inhibit ICD and, consequently, impair the effect of radiation and chemotherapy in tumours [90]. In addition to its effect in immune cells, TGF-β can also modulate the processes of epithelial-to-mesenchymal transition (EMT) in cancer cells as well as regulate the expansion of CSCs [91].
2.2.2. Targeted Therapies
In breast cancer, numerous targeted therapies have been used for a long time as the first line of treatment. These include endocrine therapies that modulate oestrogen receptor signalling [92] and, more recently, anti-HER2 therapies [93,94][93][94]. In endocrine-treated patients, a subset of fibroblasts characterised by the expression of CD146 were shown to regulate the response of cancer cells to the treatment. Briefly, CD146- fibroblasts were able to activate IGF1R tyrosine kinase signalling in the cancer cells that drove their oestrogen-independent growth, and was, therefore, hypothesised to be the cause of the irresponsiveness of cancer cells to the treatment [95]. Moreover, CAF-secreted IL-6 acted in an autocrine fashion to drive the secretion of mir221/222-loaded microvesicles, which, in its turn, induced a stem-like phenotype in cancer cells [96]. In another hormone-driven tumour type, namely prostate cancer, Zhang et al. have shown that the secretion of NRG-1 by fibroblasts could result in resistance against anti-androgen therapy [97]. We have recently shown that CAFs in luminal breast cancer can be clustered based on their expression of NRG-1 [53]. It would be interesting to understand if stromal fibroblasts expressing high-NRG-1 could be involved in modulating the response of cancer cells to hormone therapy. CAFs might also play an important role in determining the success of tumour responses to anti-HER2 therapies such as trastuzumab, since the effectiveness of this therapeutic antibody is known to depend on the induction of an immune response, similarly to chemotherapy and radiotherapy [74,98,99][74][98][99]. Due to their immunosuppressive role, CAFs can antagonize the effect of trastuzumab [100]. Additionally, the activation of fibroblast growth factor (FGF) receptor 2 (FGFR2) by CAF-derived fibroblast growth factor 5 (FGF5) results in the activation of c-Src, which bypasses the inhibition of HER2 by lapatinib, a TKI used in the treatment of HER2-positive patients [101]. The induction of the PI3K/AKT/mTOR pathway in cancer cells by fibroblasts can also promote the survival of cancer cells and impair their response to anti-HER2 kinase targeted therapies [102].
In more recent years, the advances in cancer research have provided the basis for identifying novel oncogenic vulnerabilities and molecular drivers of disease. With these advances, novel targeted therapies, including several kinase inhibitors (KI) such as BRAF inhibitors (BRAFi), EGFR inhibitors (EGFRi) and CDK4/6 inhibitors, have been introduced in the clinics [103,104,105,106][103][104][105][106] for the treatment of a variety of cancer entities. It is now increasingly evident that stromal fibroblasts are also important in dictating the outcome of cancer cells to this type of therapy. In melanoma, BRAFi can lead to an enhanced ECM deposition by fibroblasts that induced high integrin β1-FAK signalling in cancer cells. This resulted in the activation of MAPK/ERK signalling, which helped overcome the inhibitory effect of the BRAFi [64]. Hepatocyte growth factor (HGF) production by stromal fibroblasts can also drive the activation of MAPK/ERK signalling in cancer cells, which can reduce the efficacy of targeted therapy [67,107][67][107].
2.2.3. Immunotherapy
Another important component of the TME are the immune cells. Pioneering work by James Alisson [108] and Tasuko Honjo [109,110,111][109][110][111] in immune regulation has revolutionised the field of cancer immunology and established the groundwork for the development of numerous immunotherapies. Their respective findings that CTLA-4 and PD1 immune checkpoints inhibit the activity of cytotoxic T-cells and allow tumours to grow, led to the design of inhibitors against these molecules with the goal to enhance T-cell-mediated cell death of tumour cells [112]. These checkpoint inhibitors showed remarkable results, especially in melanoma patients [113,114,115][113][114][115]. However, it is not fully understood what factors dictate their efficiency and the durability of the patients’ response to these therapies. In addition to checkpoint inhibitors, several other immunotherapies are now available, including cancer vaccines [116,117][116][117] and the adoptive transfer of immune cells, such as chimeric antigen receptor (CAR) T-cells [118[118][119],119], and oncolytic viruses [120]. Due to the strong influence that fibroblasts can exert in the immune milieu of the TME [68[68][121][122],121,122], this cell type has emerged as a key modulator of the outcome of patients to these therapies. The aforementioned immunosuppressive molecules secreted by fibroblasts, namely TGF-β, IL-6, IL-8, CXCL12, can inhibit cytotoxic T-cell activity [123,124,125[123][124][125][126],126], drive myeloid-derived suppressor cell (MDSC) differentiation [127,128,129][127][128][129], modulate the phenotype of macrophages [130,131[130][131][132][133],132,133], promote Treg formation [54,134][54][134] and regulate the activity of natural killer (NK) cells [132,135][132][135]. In addition to these, further studies have described other mechanisms through which fibroblasts regulate the immune landscape in tumours. α-SMA+ CAF (myCAFs) also secrete metabolic reprogramming factors, such as IDO1, Arg2 and galectin, which are responsible for generating an immunosuppressive TME via inducing T cell anergy and inhibiting CD8+ T cell proliferation [136]. The production and deposition of ECM proteins by CAFs strongly regulate the T-cell location within the tumours [137]. A dense stroma can result in the exclusion of lymphocytes from areas rich in tumour cells, which results in poor adaptive immunity against the tumour [138,139][138][139]. Moreover, the production of certain ECM proteins by fibroblasts, namely tenascin C or thrombospondin 1 (TSP1), can negatively impact the adhesion of T-cells [140] and their activity [141] in the TME, respectively. Fibroblasts can express immune checkpoint molecules themselves, such as PD-L1 [142], PD-L2 [37,142][37][142] and B7H3 [37[37][143][144],143,144], which can all inhibit T-cell activation. The production of CCL5 by stromal fibroblasts leads to an immunosuppressive environment as a result of the recruitment of Treg cells into the TME [145]. The secretion of PGE2, which was recently shown to characterise the apCAF subpopulation, can also result in the expansion of regulatory T-cells [39]. Furthermore, PGE2 is capable of inhibiting NK cell function [146,147,148][146][147][148].
Fibroblasts not only communicate with the immune system via secreted factors, but they can also directly interact with CD8+ T-cells. The HLA-class I antigen-presentation by stromal fibroblasts along with the expression of PD-L2 and FASL results in the killing of antigen-specific cytotoxic T-cells [149]. Direct interaction between stromal fibroblasts and cancer cells also seems to drive resistance to oncolytic viruses as a result of the induction of a STING/IRF3-dependent inflammatory program in fibroblasts, which upregulates IFNβ1. The secretion of IFNβ1 into the TME induces an IFN-transcriptional program in cancer cells, rendering them less sensitive to infection by oncolytic viruses [150].
2.3. Impact of Anti-Cancer Therapies in Fibroblasts
Further evidence that supports the study and detailed investigation of fibroblast dynamics during therapy is that stromal populations are strongly modulated and influenced by a wide variety of treatments applied in the clinics, ranging from cytotoxic agents to targeted agents [151,152,153][151][152][153].
2.3.1. Direct Effects on CAFs
Most cytotoxic agents, such as chemotherapy and radiotherapy, lead to extensive DNA damage, which can result in cell cycle arrest and, consequently, senescence [154,155][154][155]. Senescence is associated with the secretion of a vast array of cytokines, chemokines and growth factors, such as CCL2, IL-6, VEGF and TGF-β, which can aid cancer cell survival and, thus, contribute to therapeutic failure [155,156,157][155][156][157]. Chan et al. showed that high doses of chemotherapy induce a potent remodelling of fibroblasts by activation of the JAK-STAT1 and NF-κB pathways, which results in the upregulation and secretion of several chemokines that, in their turn, support tumour-initiating cells and lead to chemoresistance [82]. Similarly, another study described an increased risk of developing resistance to chemotherapy after treatment with the maximum tolerated dose due to drug-induced changes in the tumour stroma [158]. In both cases, the effects observed in the tumour stroma could be prevented by adopting a metronomic (low-dose) chemotherapy regimen [82,158][82][158]. In general, chemotherapy seems to activate an inflammatory gene signature in stromal fibroblasts, which is associated with a pro-tumorigenic state [159]. The activation of the NF-κB signalling pathway by therapy-induced damage also promoted chemoresistance by driving the expression and secretion of WNT16B by fibroblasts and the subsequent activation of the Wnt program in cancer cells [160]. The secretion of interleukins (ILs) by CAFs has been reported after treatment with chemotherapy. For example, IL-17A derived from chemotherapy-treated CAFs led to the expansion of tumour-initiating cells and, consequently, was shown to contribute to therapeutic failure [63]. The exposure of fibroblasts to chemotherapy also resulted in a higher secretion of IL-11, which activated STAT3 signalling in cancer cells and drove the upregulation of anti-apoptotic pathways [161]. In addition to the effects mentioned above, chemotherapeutic agents can also drive a myCAF phenotype in stromal fibroblasts. Exposure to these drugs can increase the amount of secreted ECM by CAFs that, among other effects, can form a physical barrier and prevent the drug from reaching the cancer cells [85,162][85][162]. Chemotherapy-induced secretion of exosomes by fibroblasts can also play an important role in resistance to chemotherapy. In PDAC, Richards et al. showed that fibroblast-derived exosomes regulate EMT pathways in cancer cells in a Snail-dependent way and that targeting this pathway increased the sensitivity of cancer cells to chemotherapy [163]. Transfer of miRNAs from CAFs to cancer via secreted extracellular vesicles has also been shown to be induced by chemotherapy and to promote resistance [164].
Similar effects have been described for fibroblasts exposed to radiotherapy [151]. Strong desmoplastic reactions often characterise the irradiated areas. This is a consequence of fibroblast activation and enhanced deposition of ECM [165]. Indeed, an enrichment in αSMA-positive fibroblasts can be found in chemo and radiotherapy treated tumours [158,166][158][166] and stromal signatures are often associated with a worse outcome regarding disease free survival [166,167,168,169][166][167][168][169]. High amounts of ECM can then activate pro-survival pathways in cancer cells and impair the response to radiotherapy as well as to chemotherapy, in an integrin β1-dependent manner [87,88,170,171,172][87][88][170][171][172]. As with cytotoxic agents, radiation can drive a senescent phenotype in stromal fibroblasts, which is characterised by the expression of, among others, TGF-β [154]. The presence of elevated levels of TGF-β can then not only increase ECM production but also drive an immunosuppressive environment [154]. Tommelein et al. also found that irradiated fibroblasts could promote the survival of cancer cells via insulin-like growth factor receptor-1 (IGF1R) signalling. This pathway activation was driven by a senescence-like phenotype of the fibroblasts that was characterized by the secretion of IGF1 after treatment [173].
Despite their intended specificity, targeted therapies can also directly affect and modulate the phenotype of stromal fibroblasts. In colorectal cancer, the treatment of tumours with an EGFR inhibitor led to an increased secretion of EGF by stromal fibroblasts and conferred resistant of neighbouring cancer cells to the treatment via activation of MAPK signalling [174]. Matrix remodelling by fibroblasts exposed to BRAF inhibitors can also impair the response of cancer cells to the treatment. In this system, resistance was driven by elevated activation of integrin β1-FAK-Src signalling in malignant cells because of high ECM-production by fibroblasts [64].
2.3.2. Indirect Effects in CAFs
Fibroblasts can further be indirectly modulated by therapy, since it has been described that cancer cells acquire a specific secretory profile after exposure to drugs, named therapy-induced secretome [175]. In the same way that cytotoxic agents can drive the senescence of fibroblasts, the cancer cells exposed to these agents can also undergo therapy-induced senescence and acquire the aforementioned senescence-associated secretory phenotype (SASP) [175], which then modulates the phenotype of fibroblasts. We have recently shown that cancer cells exposed to high doses of chemotherapy upregulate the expression of IFNβ1, which acted in a paracrine manner to drive a pro-tumorigenic state of fibroblasts that then drove the recovery of cancer cells after treatment [176]. This upregulation of IFNβ1 after treatment with cytotoxic agents goes in line with previous studies that have shown that high levels of damage in cancer cells after treatment results in the activation of the STING/IRF3 pathway and drives IFNβ1 expression [177,178,179][177][178][179]. Moreover, therapies such as chemotherapy and radiation can strongly modulate the immune milieu of the TME and, consequently, affect the profile of stromal fibroblasts [74]. Radiation can result in vascular damage, which triggers an inflammatory response and, consequently, promotes myofibroblast differentiation [151]. This vascular damage can also lead to hypoxia and increase the production of HIF1α [180,181][180][181]. In colorectal cancer, it was shown that HIFα and TGFβ cooperate to induce hedgehog transcription factor GLI2 expression in tumour-initiating cancer cells, which drives stemness and chemoresistance [62]. The increased secretion of TGF-β by melanoma cells after exposure to Vemurafenib, a BRAF-inhibitor, was also able to drive fibroblast activation. Fibroblasts were then shown to produce increased levels of ECM, but also growth factors such as NRG-1 and HGF. Combined, these factors promoted the survival of cancer cells to treatment [182]. Apicella et al. described a non-autonomous cancer cell mechanism in non-small cell lung cancer (NSCLC) that drove resistance to targeted therapies. Interestingly, they show that in vivo resistant cancer cells become re-sensitized to the therapy when treated in vitro in the absence of the TME. Mechanistically, they show that cancer cells treated with TKIs targeting MET and EGFR secrete higher levels of lactate, which then instructs CAFs to secrete HGF, resulting in the non-responsiveness of cancer cells to the treatment [58].
The effects of therapies in fibroblasts are vast and complex. Despite the attempts to understand these mechanisms, further investigations are required. All the studies thus far have addressed these alterations in a bulk-fashion and detailed single-cell studies are still lacking. Moreover, it is necessary to unravel whether the mechanisms described above are tumour type- or context-specific, to develop strategies to identify which patients would benefit from their inhibition and, importantly, at what time-point during treatment. Understanding these complex dynamics is hard, but it would provide essential insights for the targeting of this cell type for cancer treatment.
2.4. Targeting Fibroblasts in Cancer Treatment
Several strategies have been developed for the targeting of fibroblasts in cancer treatment. In pre-clinical models, attempts to (1) directly target fibroblasts, (2) target secreted molecules such as ECM or soluble signalling molecules or (3) inhibit pro-tumorigenic signalling pathways have been described. Regardless of the strategy used the major goals have been to either eliminate stromal fibroblasts in general or certain subpopulations from the tumour, or to normalise the stroma/tumour-stroma interactions to ensure that the crosstalk between fibroblasts and cancer cells or the immune milieu does not support tumour progression, invasion or therapeutic failure. A summary of these strategies is described in Figure 2.
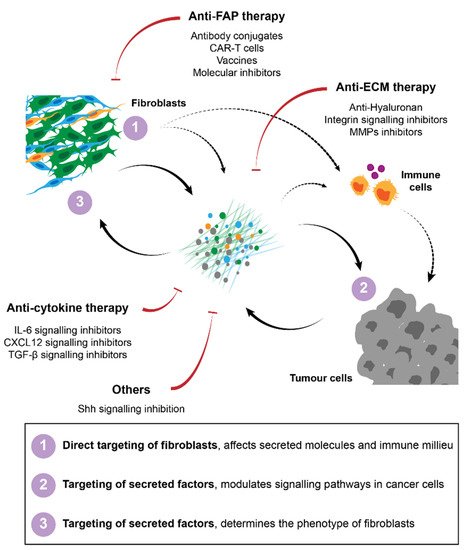

Figure 2. Strategies for cancer therapy targeting fibroblasts and their interactions in the TME. Several approaches have been proposed to target CAFs, including direct targeting and elimination of specific subpopulations of fibroblasts, namely FAP-positive cells. Therapies against secreted factors and their respective signalling, such as ECM or cytokine signalling inhibitors have also been developed to improve immunity and to block pro-tumorigenic interactions between CAFs and their surroundings.
2.4.1. Direct Targeting of Fibroblasts
Numerous attempts of targeting fibroblasts have focused on their direct targeting via specific cell surface markers. Some early studies in PDAC have targeted αSMA-expressing fibroblasts. However, contrary to what had been expected, tumours in which this stromal population was eliminated grew at even higher rates, demonstrating that, at least in this tumour type, αSMA-positive fibroblasts restricted tumour growth [28,29][28][29]. Similar attempts to eliminate or directly target fibroblasts in the TME have instead focused on the FAP expressing fibroblast population. In this case, regression of the tumour was indeed observed [123,129][123][129]. Diverse FAP-targeting strategies have since been developed and tested in pre-clinical models, including genetic deletion [123[123][129],129], molecular inhibitors that block FAP enzymatic activity [129[129][183][184][185][186],183,184,185,186], anti-FAP monoclonal antibodies [187] and FAP-antigen vaccination [188], all with promising tumour-restraining results. Most of these studies correlate the observed effects with the impact of the treatment in the immune milieu. The tumours in which FAP was targeted were characterised by a reversion of the immunosuppressive environment and an increase in T-cell infiltration [123,185,186][123][185][186]. Consequently, clinical trials targeting this protein have been undertaken to investigate the potential of such treatments in patients. The usage of blocking antibodies, namely sibrotuzumab (a humanised anti-FAP antibody, F19), proved to be safe in a phase I trial [189[189][190],190], but failed to significantly improve overall survival. Similar results were obtained with the FAP-inhibitor PT100 (talabostat) [191,192,193,194][191][192][193][194]. Even though the application of anti-FAP antibodies showed very limited clinical efficacy, these agents exhibited very good stroma-targeting properties [190]. This has led to the development of antibody conjugates, in which FAP is used for the localised delivery of the conjugate. One such fusion antibody that is currently in a phase I clinical trial (NCT02627274) is called RO6874281, in which FAP is cross-linked with IL-2. The main goal of using this antibody is to activate T-cells in the TME [195]. Other strategies involve the conjugation of FAP with other immune modulators, such as IL15 [196], co-stimulatory ligands as B7.2 [197] and CD40 [198], or immunotoxins [199,200][199][200]. Moreover, FAP antibodies can be conjugated with agents that will directly induce apoptosis, such as the cytotoxic drug DM1, which have shown potent inhibitory activity in pre-clinical models [187]. Finally, the ablation of FAP-expressing cells in the TME was also achieved by using CAR-T cells directed against FAP [201,202][201][202].
In summary, a broad variety of strategies targeting FAP are available, although clinical trial results have been disappointing thus far. This might be improved with optimised strategies to directly target and eliminate FAP-positive cells, such as conjugated antibodies, vaccination or bispecific CAR-T cells. The enhanced immune response that has been observed in the pre-clinical models suggests that a combination of these strategies with other therapies, such as immune checkpoint inhibitors or chemotherapy, will likely be advantageous. Moreover, several studies clearly showed that these approaches are highly effective to target specific conjugates to the tumour site, which can be further explored.
Another cell surface marker that has been explored in pre-clinical models is GPR77. This protein, in combination with CD10, has been shown to identify a subpopulation of fibroblasts that is responsible for driving chemoresistance. The blockage of GPR77 using a neutralising antibody was shown to reduce the CSCs in tumours and enhance the response to chemotherapy [60]. Moreover, since these fibroblasts were identified as being present in the tumour prior to treatment, the concomitant targeting of these with the chemotherapeutic regiments could likely provide a benefit for the patient. Further studies along these lines are required.
2.4.2. Targeting of the ECM
Stromal rich tumours can have impaired drug delivery as a result of the physical barrier presented by the ECM. Moreover, the ECM can activate pro-survival signalling pathways in tumour cells. This makes approaches targeting the ECM and/or its downstream signalling attractive for the treatment of a variety of tumours. Numerous pre-clinical studies blocking integrin signalling have shown that this axis is critical for the development of drug resistance and that its abrogation in combination with cytotoxic agents could improve therapy response and overall survival [87,203][87][203]. A blocking antibody, FG-3019, that interferes with integrin signalling activation is being tested in a phase I/II clinical trial (NCT02210559). Moreover, the biosafety of defactinib, a small molecule inhibitor that targets FAK and thereby prevents downstream pathway activation in cancer cells, has been investigated in a phase I clinical trial (NCT02546531) [204]. Direct targeting of ECM proteins, namely fibronectin [205], tenascin C [206] and hyaluronan [138,207,208][138][207][208] have also been studied in pre-clinical models with promising results. The targeting of hyaluronan for degradation using a PEGylated enzyme, PEGPH20, in combination with chemotherapy showed positive results in early phase clinical trial in patients with PDAC [209,210][209][210]. Losartan, a small molecule inhibitor that targets the angiotensin receptor and, consequently, leads to a decrease in hyaluronan levels, is currently tested in a phase I clinical trial (NCT03563248) [211]. Another strategy employed in pre-clinical trials exploits the inhibition of matrix metalloproteinases (MMPs). These enzymes are critical players in the remodelling of the ECM [212]. Unfortunately, disappointing results have emerged from all the MMP inhibitors tested in clinical trials thus far [213]. Novel strategies are also emerging, with the use of CAR-T cells engineered to express heparanase, an enzyme that degrades ECM proteins, having shown promising pre-clinical results by enhancing T cell infiltration and anti-tumour activity [214].
2.4.3. Targeting of Cytokines and Growth Factors
One of the most extensively studied and described pro-tumorigenic axes involved in the communication between cancer cells and their microenvironment is the IL-6/IL-6R/JAK-STAT3 pathway. Not surprisingly, attempts to target this pathway soon emerged and were tested in pre-clinical models [215]. The targeting of this axis is threefold, since (1) it has been described to be involved in the activation of fibroblasts [216,217][216][217], (2) its effect in tumour cells drives pro-tumorigenic states by, among others, modulating stemness and invasiveness [218] and (3) it negatively regulates tumour-infiltrating immune cells [219]. An IL-6 monoclonal antibody, siltuximab, was tested in a phase II clinical trial for the treatment of prostate cancer and demonstrated a safe profile but failed to improve patient outcome [220]. The efficacy of this antibody is currently evaluated in a phase Ib/II trial for the treatment of advanced pancreatic cancer (NCT04191421). Furthermore, several clinical trials investigating the activity of molecules that inhibit downstream signalling of IL6-, namely ROCK and STAT3 inhibitors, have been undertaken. A dual ROCK-AKT inhibitor, AT13148, was tested in phase I, but failed to show a safe profile [221]. The STAT3 inhibitor, AZD9150, was well tolerated in a phase I clinical trial [222] and an investigation of its efficacy is undergoing.
Another major pro-tumorigenic molecule that is secreted by CAFs is CXCL12. This chemokine is mostly involved in the modulation of immune cells in the TME, and its targeting can alleviate immunosuppression and drive effective anti-tumour immunity. Therefore, the inhibition of this axis using antagonists or antibodies targeting its receptor, CXCR4, has been extensively studied [223,224][223][224]. The efficacy of some of these molecules, namely the CXCR4 antagonist AMD3100, is currently evaluated in clinical trials for the treatment of several tumour entities, including haematological malignancies and advanced pancreatic cancer (https://www.cancer.gov/about-cancer/treatment/clinical-trials/intervention/plerixafor, accessed on 1 June 2021), after having displayed a safe profile in phase I clinical trials [225,226][225][226].
The inhibition of Shh and TGFβ has also been extensively studied in an attempt to ‘normalise’ the tumour stroma by reverting the activated state of fibroblasts. Moreover, these molecules have broad activities and are capable of regulating several other processes, as described in the previous sections. Despite the exciting results of Shh inhibition in pre-clinical studies [52[52][61][227],61,227], evaluation of the FDA-approved inhibitors, saridegib and vismodegib, in early phase clinical trials showed very disappointing results with no improvement in disease-free survival (DFS) and overall survival (OS) (NCT01064622; NCT01088815) [228]. To date, several TGFβ inhibitors have been developed and their efficacy studied using pre-clinical models [229,230,231][229][230][231]. Following these results, clinical trials have been established and, while some have failed to demonstrate a significant overall benefit, other trials showed a benefit of inhibiting TGF-β signalling [231].
Despite the many attempts to target the TME and to significantly improve patient outcomes, most of these strategies have fallen short. Improved combinatorial strategies will likely be required to achieve an effective inhibition of tumour growth. Moreover, most of the clinical trials enrol patients with heavily pre-treated tumours and advanced/metastatic disease, which can hinder the effectiveness of these drugs. The rational targeting of these axes in early treatment could provide an advantage by preventing the development of resistance and inhibiting the invasive properties in cancer cells. However, more studies are required to evaluate this hypothesis. Furthermore, increasing knowledge in CAF biology and the unravelling of subpopulation-specific markers will likely drive the development of more effective treatments as this might allow the targeting of particularly relevant subpopulations with enhanced specificity. Finally, the identification of biomarkers and the better stratification of patients will be necessary to see a potential improvement in disease-free survival and overall survival in particular patient groups, as with other targeted therapies in cancer therapy.
References
- Schmidt, A.; Weber, O.F. Memoriam of Rudolf Virchow: A Historical Retrospective Including Aspects of Inflammation, Infection and Neoplasia; Contributions to Microbiology; KARGER: Basel, Switzerland, 2006; Volume 13, pp. 1–15.
- Paget, S. The Distribution of Secondary Growths in Cancer of the Breast. 1889. Cancer Metastasis Rev. 1989, 8, 98–101.
- Maman, S.; Witz, I.P. A history of exploring cancer in context. Nat. Rev. Cancer 2018, 18, 359–376.
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598.
- Marsh, T.; Pietras, K.; McAllister, S.S. Fibroblasts as architects of cancer pathogenesis. BBA Mol. Basis Disease 2013, 1832, 1070–1078.
- Dvorak, H.F. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986, 315, 1650–1659.
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401.
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363.
- Foster, D.S.; Jones, R.E.; Ransom, R.C.; Longaker, M.T.; Norton, J.A. The evolving relationship of wound healing and tumor stroma. JCI Insight 2018, 3, 696.
- Baslan, T.; Hicks, J. Unravelling biology and shifting paradigms in cancer with single-cell sequencing. Nat. Rev. Cancer 2017, 17, 557–569.
- Virchow, R. Die Cellularpathologie in ihrer Begründung auf physiologische und pathologische Gewebelehre. 1871. Available online: (accessed on 1 May 2021).
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123.
- Gabbiani, G.; Ryan, G.B.; Majne, G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971, 27, 549–550.
- Darby, I.; Skalli, O.; Gabbiani, G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab. Investig. 1990, 63, 21–29.
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Trans. Med. 2014, 6, 265sr6.
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186.
- Cheng, F.; Shen, Y.; Mohanasundaram, P.; Lindström, M.; Ivaska, J.; Ny, T.; Eriksson, J.E. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-Slug signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E4320–E4327.
- Strutz, F.; Okada, H.; Lo, C.W.; Danoff, T.; Carone, R.L.; Tomaszewski, J.E.; Neilson, E.G. Identification and characterization of a fibroblast marker: FSP1. J. Cell Biol. 1995, 130, 393–405.
- Österreicher, C.H.; Penz-Österreicher, M.; Grivennikov, S.I.; Guma, M.; Koltsova, E.K.; Datz, C.; Sasik, R.; Hardiman, G.; Karin, M.; Brenner, D.A. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc. Natl. Acad. Sci. USA 2011, 108, 308–313.
- Farahani, R.M.; Xaymardan, M. Platelet-Derived Growth Factor Receptor Alpha as a Marker of Mesenchymal Stem Cells in Development and Stem Cell Biology. Stem. Cells Int. 2015, 2015, 362753.
- Desmouliere, A.; Guyot, C.; Gabbiani, G. The stroma reaction myofibroblast: A key player in the control of tumor cell behavior. Int. J. Dev. Biol. 2004, 48, 509–517.
- Garin-Chesa, P.; Old, L.J.; Rettig, W.J. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc. Natl. Acad. Sci. USA 1990, 87, 7235–7239.
- LeBleu, V.S.; Neilson, E.G. Origin and functional heterogeneity of fibroblasts. FASEB J. 2020, 34, 3519–3536.
- Shaw, T.J.; Rognoni, E. Dissecting Fibroblast Heterogeneity in Health and Fibrotic Disease. Curr. Rheumatol. Rep. 2020, 22, 33.
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277–281.
- Vorstandlechner, V.; Laggner, M.; Kalinina, P.; Haslik, W.; Radtke, C.; Shaw, L.; Lichtenberger, B.M.; Tschachler, E.; Ankersmit, H.J.; Mildner, M. Deciphering the functional heterogeneity of skin fibroblasts using single-cell RNA sequencing. FASEB J. 2020, 34, 3677–3692.
- Burrell, R.A.; McGranahan, N.; Bartek, J.; Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 2013, 501, 338–345.
- Özdemir, B.C.; Pentcheva-Hoang, T.; Carstens, J.L.; Zheng, X.; Wu, C.-C.; Simpson, T.R.; Laklai, H.; Sugimoto, H.; Kahlert, C.; Novitskiy, S.V.; et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014, 25, 719–734.
- Rhim, A.D.; Oberstein, P.E.; Thomas, D.H.; Mirek, E.T.; Palermo, C.F.; Sastra, S.A.; Dekleva, E.N.; Saunders, T.; Becerra, C.P.; Tattersall, I.W.; et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014, 25, 735–747.
- Yang, X.; Lin, Y.; Shi, Y.; Li, B.; Liu, W.; Yin, W.; Dang, Y.; Chu, Y.; Fan, J.; He, R. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3-CCL2 Signaling. Cancer Res. 2016, 76, 4124–4135.
- Zhang, Y.; Ertl, H.C.J. Depletion of FAP+ cells reduces immunosuppressive cells and improves metabolism and functions CD8+T cells within tumors. Oncotarget 2016, 7, 23282–23299.
- Fearon, D.T. The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol. Res. 2014, 2, 187–193.
- Zheng, G.X.Y.; Terry, J.M.; Belgrader, P.; Ryvkin, P.; Bent, Z.W.; Wilson, R.; Ziraldo, S.B.; Wheeler, T.D.; McDermott, G.P.; Zhu, J.; et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 2017, 8, 14049.
- Kong, W.; Biddy, B.A.; Kamimoto, K.; Amrute, J.M.; Butka, E.G.; Morris, S.A. CellTagging: Combinatorial indexing to simultaneously map lineage and identity at single-cell resolution. Nat. Protoc. 2020, 15, 750–772.
- Bartoschek, M.; Oskolkov, N.; Bocci, M.; Lövrot, J.; Larsson, C.; Sommarin, M.; Madsen, C.D.; Lindgren, D.; Pekar, G.; Karlsson, G.; et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 2018, 9, 5150.
- Friedman, G.; Levi-Galibov, O.; David, E.; Bornstein, C.; Giladi, A.; Dadiani, M.; Mayo, A.; Halperin, C.; Pevsner-Fischer, M.; Lavon, H.; et al. Cancer-associated fibroblast compositions change with breast cancer progression linking the ratio of S100A4 + and PDPN + CAFs to clinical outcome. Nat. Cancer 2020, 1, 692–708.
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018, 33, 463–479.e10.
- Öhlund, D.; Handly-Santana, A.; Biffi, G.; Elyada, E.; Almeida, A.S.; Ponz-Sarvise, M.; Corbo, V.; Oni, T.E.; Hearn, S.A.; Lee, E.J.; et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017, 214, 579–596.
- Elyada, E.; Bolisetty, M.; Laise, P.; Flynn, W.F.; Courtois, E.T.; Burkhart, R.A.; Teinor, J.A.; Belleau, P.; Biffi, G.; Lucito, M.S. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Disc. 2019, 9, 1102–1123.
- Hosein, A.N.; Huang, H.; Wang, Z.; Parmar, K.; Du, W.; Huang, J.; Maitra, A.; Olson, E.; Verma, U.; Brekken, R.A. Cellular heterogeneity during mouse pancreatic ductal adenocarcinoma progression at single-cell resolution. JCI Insight 2019, 4, e129212.
- Neuzillet, C.; Tijeras-Raballand, A.; Ragulan, C.; Cros, J.; Patil, Y.; Martinet, M.; Erkan, M.; Kleeff, J.; Wilson, J.; Apte, M.; et al. Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J. Pathol. 2019, 248, 51–65.
- Bernard, V.; Semaan, A.; Huang, J.; San Lucas, F.A.; Mulu, F.C.; Stephens, B.M.; Guerrero, P.A.; Huang, Y.; Zhao, J.; Kamyabi, N.; et al. Single-Cell Transcriptomics of Pancreatic Cancer Precursors Demonstrates Epithelial and Microenvironmental Heterogeneity as an Early Event in Neoplastic Progression. Clin. Cancer Res. 2019, 25, 2194–2205.
- Li, H.; Courtois, E.T.; Sengupta, D.; Tan, Y.; Chen, K.H.; Goh, J.J.L.; Kong, S.L.; Chua, C.; Hon, L.K.; Tan, W.S.; et al. Author Correction: Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat. Genet. 2018, 50, 1754.
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 2017, 171, 1611–1624.e24.
- Davidson, S.; Efremova, M.; Riedel, A.; Mahata, B.; Pramanik, J.; Huuhtanen, J.; Kar, G.; Vento-Tormo, R.; Hagai, T.; Chen, X.; et al. Single-Cell RNA Sequencing Reveals a Dynamic Stromal Niche That Supports Tumor Growth. CellReports 2020, 31, 107628.
- Chen, Z.; Zhou, L.; Liu, L.; Hou, Y.; Xiong, M.; Yang, Y.; Hu, J.; Chen, K. Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat. Comms. 2020, 11, 5077–5112.
- Patel, A.K.; Vipparthi, K.; Thatikonda, V.; Arun, I.; Bhattacharjee, S.; Sharan, R.; Arun, P.; Singh, S. A subtype of cancer-associated fibroblasts with lower expression of alpha-smooth muscle actin suppresses stemness through BMP4 in oral carcinoma. Oncogenesis 2018, 7, 78.
- Hornburg, M.; Desbois, M.; Lu, S.; Guan, Y.; Lo, A.A.; Kaufman, S.; Elrod, A.; Lotstein, A.; DesRochers, T.M.; Munoz-Rodriguez, J.L.; et al. Single-cell dissection of cellular components and interactions shaping the tumor immune phenotypes in ovarian cancer. Cancer Cell 2021, 39, 928–944.
- Affo, S.; Nair, A.; Brundu, F.; Ravichandra, A.; Bhattacharjee, S.; Matsuda, M.; Chin, L.; Filliol, A.; Wen, W.; Song, X.; et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell 2021, 39, 866–882.
- Sebastian, A.; Hum, N.R.; Martin, K.A.; Gilmore, S.F.; Peran, I.; Byers, S.W.; Wheeler, E.K.; Coleman, M.A.; Loots, G.G. Single-Cell Transcriptomic Analysis of Tumor-Derived Fibroblasts and Normal Tissue-Resident Fibroblasts Reveals Fibroblast Heterogeneity in Breast Cancer. Cancers 2020, 12, 1307.
- Biffi, G.; Oni, T.E.; Spielman, B.; Hao, Y.; Elyada, E.; Park, Y.; Preall, J.; Tuveson, D.A. IL1-Induced JAK/STAT Signaling Is Antagonized by TGFβ to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discov. 2019, 9, 282–301.
- Steele, N.G.; Biffi, G.; Kemp, S.B.; Zhang, Y.; Drouillard, D.; Syu, L.; Hao, Y.; Oni, T.E.; Brosnan, E.; Elyada, E.; et al. Inhibition of Hedgehog Signaling Alters Fibroblast Composition in Pancreatic Cancer. Clin. Cancer Res. 2021, 27, 2023–2037.
- Berdiel-Acer, M.; Maia, A.; Hristova, Z.; Borgoni, S.; Vetter, M.; Burmester, S.; Becki, C.; Michels, B.; Abnaof, K.; Binenbaum, I.; et al. Stromal NRG1 in luminal breast cancer defines pro-fibrotic and migratory cancer-associated fibroblasts. Oncogene 2021, 40, 2651–2666.
- Kieffer, Y.; Hocine, H.R.; Gentric, G.; Pelon, F.; Bernard, C.; Bourachot, B.; Lameiras, S.; Albergante, L.; Bonneau, C.; Guyard, A.; et al. Single-Cell Analysis Reveals Fibroblast Clusters Linked to Immunotherapy Resistance in Cancer. Cancer Discov. 2020, 10, 1330–1351.
- Gieniec, K.A.; Butler, L.M.; Worthley, D.L.; Woods, S.L. Cancer-associated fibroblasts-heroes or villains? Br. J. Cancer 2019, 121, 293–302.
- Longley, D.B.; Johnston, P.G. Molecular mechanisms of drug resistance. J. Pathol. 2005, 205, 275–292.
- Teicher, B.A.; Herman, T.S.; Holden, S.A.; Wang, Y.Y.; Pfeffer, M.R.; Crawford, J.W.; Frei, E. Tumor resistance to alkylating agents conferred by mechanisms operative only in vivo. Science 1990, 247, 1457–1461.
- Apicella, M.; Giannoni, E.; Fiore, S.; Ferrari, K.J.; Fernández-Pérez, D.; Isella, C.; Granchi, C.; Minutolo, F.; Sottile, A.; Comoglio, P.M.; et al. Increased Lactate Secretion by Cancer Cells Sustains Non-cell-autonomous Adaptive Resistance to MET and EGFR Targeted Therapies. Cell Metab. 2018, 28, 848–865.e6.
- Boelens, M.C.; Wu, T.J.; Nabet, B.Y.; Xu, B.; Qiu, Y.; Yoon, T.; Azzam, D.J.; Twyman-Saint Victor, C.; Wiemann, B.Z.; Ishwaran, H.; et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell 2014, 159, 499–513.
- Su, S.; Chen, J.; Yao, H.; Liu, J.; Yu, S.; Lao, L.; Wang, M.; Luo, M.; Xing, Y.; Chen, F.; et al. CD10+GPR77+ Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell 2018, 172, 841–856.e16.
- Valenti, G.; Quinn, H.M.; Heynen, G.J.J.E.; Lan, L.; Holland, J.D.; Vogel, R.; Wulf-Goldenberg, A.; Birchmeier, W. Cancer Stem Cells Regulate Cancer-Associated Fibroblasts via Activation of Hedgehog Signaling in Mammary Gland Tumors. Cancer Res. 2017, 77, 2134–2147.
- Tang, Y.-A.; Chen, Y.-F.; Bao, Y.; Mahara, S.; Yatim, S.M.J.M.; Oguz, G.; Lee, P.L.; Feng, M.; Cai, Y.; Tan, E.Y.; et al. Hypoxic tumor microenvironment activates GLI2 via HIF-1α and TGF-β2 to promote chemoresistance in colorectal cancer. Proc. Natl. Acad. Sci. USA 2018, 115, E5990–E5999.
- Lotti, F.; Jarrar, A.M.; Pai, R.K.; Hitomi, M.; Lathia, J.; Mace, A.; Gantt, G.A.; Sukhdeo, K.; DeVecchio, J.; Vasanji, A.; et al. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J. Exp. Med. 2013, 210, 2851–2872.
- Hirata, E.; Girotti, M.R.; Viros, A.; Hooper, S.; Spencer-Dene, B.; Matsuda, M.; Larkin, J.; Marais, R.; Sahai, E. Intravital Imaging Reveals How BRAF Inhibition Generates Drug-Tolerant Microenvironments with High Integrin β1/FAK Signaling. Cancer Cell 2015, 27, 574–588.
- Shien, K.; Papadimitrakopoulou, V.A.; Ruder, D.; Behrens, C.; Shen, L.; Kalhor, N.; Song, J.; Lee, J.J.; Wang, J.; Tang, X.; et al. JAK1/STAT3 activation through a proinflammatory cytokine pathway leads to resistance to molecularly targeted therapy in non-small cell lung cancer. Mol. Cancer Ther. 2017, 16, 2234–2245.
- Ishibashi, M.; Neri, S.; Hashimoto, H.; Miyashita, T.; Yoshida, T.; Nakamura, Y.; Udagawa, H.; Kirita, K.; Matsumoto, S.; Umemura, S.; et al. CD200-positive cancer associated fibroblasts augment the sensitivity of Epidermal Growth Factor Receptor mutation-positive lung adenocarcinomas to EGFR Tyrosine kinase inhibitors. Sci. Rep. 2017, 7, 46662.
- Straussman, R.; Morikawa, T.; Shee, K.; Barzily-Rokni, M.; Qian, Z.R.; Du, J.; Davis, A.; Mongare, M.M.; Gould, J.; Frederick, D.T.; et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 2012, 487, 500–504.
- Monteran, L.; Erez, N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front. Immunol. 2019, 10, 1835.
- Altrock, P.M.; Yoon, N.; Bull, J.A.; Wu, H.; Ruiz-Ramírez, J.; Miroshnychenko, D.; Kimmel, G.J.; Kim, E.; Vander Velde, R.J.; Rejniak, K.; et al. The impact of tumor stromal architecture on therapy response and clinical progression. bioRxiv 2018, 451047.
- Qin, X.; Sufi, J.; Vlckova, P.; Kyriakidou, P.; Acton, S.E.; Li, V.S.W.; Nitz, M.; Tape, C.J. Cell-type-specific signaling networks in heterocellular organoids. Nat. Methods 2020, 17, 335–342.
- Sistigu, A.; Yamazaki, T.; Vacchelli, E.; Chaba, K.; Enot, D.P.; Adam, J.; Vitale, I.; Goubar, A.; Baracco, E.E.; Remédios, C.; et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat. Med. 2014, 20, 1301–1309.
- Burnette, B.C.; Liang, H.; Lee, Y.; Chlewicki, L.; Khodarev, N.N.; Weichselbaum, R.R.; Fu, Y.-X.; Auh, S.L. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res 2011, 71, 2488–2496.
- Gupta, A.; Probst, H.C.; Vuong, V.; Landshammer, A.; Muth, S.; Yagita, H.; Schwendener, R.; Pruschy, M.; Knuth, A.; van den Broek, M. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J. Immunol. 2012, 189, 558–566.
- Kroemer, G.; Senovilla, L.; Galluzzi, L.; André, F.; Zitvogel, L. Natural and therapy-induced immunosurveillance in breast cancer. Nat. Med. 2015, 21, 1128–1138.
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72.
- Nguyen, L.V.; Vanner, R.; Dirks, P.; Eaves, C.J. Cancer stem cells: An evolving concept. Nat. Rev. Cancer 2012, 12, 133–143.
- Schulz, A.; Meyer, F.; Dubrovska, A.; Borgmann, K. Cancer Stem Cells and Radioresistance: DNA Repair and Beyond. Cancers 2019, 11, 862.
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134.
- Kanzaki, R.; Pietras, K. Heterogeneity of cancer-associated fibroblasts: Opportunities for precision medicine. Cancer Sci. 2020, 111, 2708–2717.
- Khodarev, N.N.; Beckett, M.; Labay, E.; Darga, T.; Roizman, B.; Weichselbaum, R.R. STAT1 is overexpressed in tumors selected for radioresistance and confers protection from radiation in transduced sensitive cells. Proc. Natl. Acad. Sci. USA 2004, 101, 1714–1719.
- Weichselbaum, R.R.; Ishwaran, H.; Yoon, T.; Nuyten, D.S.A.; Baker, S.W.; Khodarev, N.; Su, A.W.; Shaikh, A.Y.; Roach, P.; Kreike, B.; et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc. Natl. Acad. Sci. USA 2008, 105, 18490–18495.
- Chan, T.-S.; Hsu, C.-C.; Pai, V.C.; Liao, W.-Y.; Huang, S.-S.; Tan, K.-T.; Yen, C.-J.; Hsu, S.-C.; Chen, W.-Y.; Shan, Y.-S.; et al. Metronomic chemotherapy prevents therapy-induced stromal activation and induction of tumor-initiating cells. J. Exp. Med. 2016, 213, 2967–2988.
- Hu, Y.-B.; Yan, C.; Mu, L.; Mi, Y.-L.; Zhao, H.; Hu, H.; Li, X.-L.; Tao, D.-D.; Wu, Y.-Q.; Gong, J.-P.; et al. Correction: Exosomal Wnt-induced dedifferentiation of colorectal cancer cells contributes to chemotherapy resistance. Oncogene 2019, 38, 6319–6321.
- Seino, T.; Kawasaki, S.; Shimokawa, M.; Tamagawa, H.; Toshimitsu, K.; Fujii, M.; Ohta, Y.; Matano, M.; Nanki, K.; Kawasaki, K.; et al. Human Pancreatic Tumor Organoids Reveal Loss of Stem Cell Niche Factor Dependence during Disease Progression. Cell Stem Cell 2018, 22, 454–467.e6.
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D.; et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009, 324, 1457–1461.
- Perentes, J.Y.; McKee, T.D.; Ley, C.D.; Mathiew, H.; Dawson, M.; Padera, T.P.; Munn, L.L.; Jain, R.K.; Boucher, Y. In vivo imaging of extracellular matrix remodeling by tumor-associated fibroblasts. Nat. Methods 2009, 6, 143–145.
- Park, C.C.; Zhang, H.J.; Yao, E.S.; Park, C.J.; Bissell, M.J. Beta1 integrin inhibition dramatically enhances radiotherapy efficacy in human breast cancer xenografts. Cancer Res 2008, 68, 4398–4405.
- Mantoni, T.S.; Lunardi, S.; Al-Assar, O.; Masamune, A.; Brunner, T.B. Pancreatic stellate cells radioprotect pancreatic cancer cells through β1-integrin signaling. Cancer Res 2011, 71, 3453–3458.
- Uchihara, T.; Miyake, K.; Yonemura, A.; Komohara, Y.; Itoyama, R.; Koiwa, M.; Yasuda, T.; Arima, K.; Harada, K.; Eto, K.; et al. Extracellular Vesicles from Cancer-Associated Fibroblasts Containing Annexin A6 Induces FAK-YAP Activation by Stabilizing β1 Integrin, Enhancing Drug Resistance. Cancer Res. 2020, 80, 3222–3235.
- Vanpouille-Box, C.; Diamond, J.M.; Pilones, K.A.; Zavadil, J.; Babb, J.S.; Formenti, S.C.; Barcellos-Hoff, M.H.; Demaria, S. TGFβ Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity. Cancer Res 2015, 75, 2232–2242.
- Bellomo, C.; Caja, L.; Moustakas, A. Transforming growth factor β as regulator of cancer stemness and metastasis. Br. J. Cancer 2016, 115, 761–769.
- Pondé, N.F.; Zardavas, D.; Piccart, M. Progress in adjuvant systemic therapy for breast cancer. Nat. Rev. Clin. Oncol. 2019, 16, 27–44.
- Dillman, R.O. Perceptions of Herceptin: A monoclonal antibody for the treatment of breast cancer. Cancer Biother. Radiopharm. 1999, 14, 5–10.
- Cortes, J.; Saura, C.; Bellet, M.; Muñoz-Couselo, E.; Ramírez-Merino, N.; Calvo, V.; Pérez, J.; Vidal, M. HER2 and hormone receptor-positive breast cancer--blocking the right target. Nat. Rev. Clin. Oncol. 2011, 8, 307–311.
- Brechbuhl, H.M.; Finlay-Schultz, J.; Yamamoto, T.M.; Gillen, A.E.; Cittelly, D.M.; Tan, A.-C.; Sams, S.B.; Pillai, M.M.; Elias, A.D.; Robinson, W.A.; et al. Fibroblast Subtypes Regulate Responsiveness of Luminal Breast Cancer to Estrogen. Clin. Cancer Res. 2017, 23, 1710–1721.
- Sansone, P.; Berishaj, M.; Rajasekhar, V.K.; Ceccarelli, C.; Chang, Q.; Strillacci, A.; Savini, C.; Shapiro, L.; Bowman, R.L.; Mastroleo, C.; et al. Evolution of Cancer Stem-like Cells in Endocrine-Resistant Metastatic Breast Cancers Is Mediated by Stromal Microvesicles. Cancer Res. 2017, 77, 1927–1941.
- Zhang, Z.; Karthaus, W.R.; Lee, Y.S.; Gao, V.R.; Wu, C.; Russo, J.W.; Liu, M.; Mota, J.M.; Abida, W.; Linton, E.; et al. Tumor Microenvironment-Derived NRG1 Promotes Antiandrogen Resistance in Prostate Cancer. Cancer Cell 2020, 38, 279–296.e9.
- Triulzi, T.; Regondi, V.; De Cecco, L.; Cappelletti, M.R.; Di Modica, M.; Paolini, B.; Lollini, P.L.; Di Cosimo, S.; Sfondrini, L.; Generali, D.; et al. Early immune modulation by single-agent trastuzumab as a marker of trastuzumab benefit. Br. J. Cancer 2018, 119, 1487–1494.
- Griguolo, G.; Pascual, T.; Dieci, M.V.; Guarneri, V.; Prat, A. Interaction of host immunity with HER2-targeted treatment and tumor heterogeneity in HER2-positive breast cancer. J. Immunother. Cancer 2019, 7, 90.
- Nguyen, M.; De Ninno, A.; Mencattini, A.; Mermet-Meillon, F.; Fornabaio, G.; Evans, S.S.; Cossutta, M.; Khira, Y.; Han, W.; Sirven, P.; et al. Dissecting Effects of Anti-cancer Drugs and Cancer-Associated Fibroblasts by On-Chip Reconstitution of Immunocompetent Tumor Microenvironments. Cell Rep. 2018, 25, 3884–3893.e3.
- Fernandez-Nogueira, P.; Mancino, M.; Fuster, G.; Lopez-Plana, A.; Jauregui, P.; Almendro, V.; Enreig, E.; Menéndez, S.; Rojo, F.; Noguera-Castells, A.; et al. Tumor-Associated Fibroblasts Promote HER2-Targeted Therapy Resistance through FGFR2 Activation. Clin. Cancer Res. 2019, 26, 1432–1448.
- Zervantonakis, I.K.; Poskus, M.D.; Scott, A.L.; Selfors, L.M.; Lin, J.-R.; Dillon, D.A.; Pathania, S.; Sorger, P.K.; Mills, G.B.; Brugge, J.S. Fibroblast-tumor cell signaling limits HER2 kinase therapy response via activation of MTOR and antiapoptotic pathways. Proc. Natl. Acad. Sci. USA 2020, 117, 16500–16508.
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. BRIM-3 Study Group Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516.
- Flaherty, K.T.; Puzanov, I.; Kim, K.B.; Ribas, A.; McArthur, G.; Sosman, J.A.; O’Dwyer, P.J.; Lee, R.J.; Grippo, J.F.; Nolop, K.; et al. Inhibition of Mutated, Activated BRAF in Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 809–819.
- Shepherd, F.A.; Rodrigues Pereira, J.; Ciuleanu, T.; Tan, E.H.; Hirsh, V.; Thongprasert, S.; Campos, D.; Maoleekoonpiroj, S.; Smylie, M.; Martins, R.; et al. National Cancer Institute of Canada Clinical Trials Group Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 2005, 353, 123–132.
- Finn, R.S.; Crown, J.P.; Láng, I.; Boer, K.; Bondarenko, I.M.; Kulyk, S.O.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015, 16, 25–35.
- Wang, W.; Li, Q.; Yamada, T.; Matsumoto, K.; Matsumoto, I.; Oda, M.; Watanabe, G.; Kayano, Y.; Nishioka, Y.; Sone, S.; et al. Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase in-hibitors. Clin. Cancer Res. 2009, 15, 6630–6638.
- Leach, D.R.; Krummel, M.F.; Allison, J. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736.
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895.
- Nishimura, H.; Minato, N.; Nakano, T.; Honjo, T. Immunological studies on PD-1 deficient mice: Implication of PD-1 as a negative regulator for B cell responses. Int. Immunol. 1998, 10, 1563–1572.
- Nishimura, H.; Honjo, T. PD-1: An inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001, 22, 265–268.
- Hoos, A. Development of immuno-oncology drugs-from CTLA4 to PD1 to the next generations. Nat. Rev. Drug Discov. 2016, 15, 235–247.
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723.
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015, 16, 908–918.
- Schadendorf, D.; Hodi, F.S.; Robert, C.; Weber, J.S.; Margolin, K.; Hamid, O.; Patt, D.; Chen, T.-T.; Berman, D.M.; Wolchok, J.D. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2015, 33, 1889–1894.
- Melief, C.J.M.; van Hall, T.; Arens, R.; Ossendorp, F.; van der Burg, S.H. Therapeutic cancer vaccines. J. Clin. Invest. 2015, 125, 3401–3412.
- Hu, Z.; Ott, P.A.; Wu, C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 2018, 18, 168–182.
- Rosenberg, S.A.; Restifo, N.P.; Yang, J.C.; Morgan, R.A.; Dudley, M.E. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 2008, 8, 299–308.
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365.
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Erratum: Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2016, 15, 660.
- Barrett, R.L.; Puré, E. Cancer-associated fibroblasts and their influence on tumor immunity and immunotherapy. eLife 2020, 9, 9.
- Liu, T.; Han, C.; Wang, S.; Fang, P.; Ma, Z.; Xu, L.; Yin, R. Cancer-associated fibroblasts: An emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 2019, 12, 1–15.
- Feig, C.; Jones, J.O.; Kraman, M.; Wells, R.J.B.; Deonarine, A.; Chan, D.S.; Connell, C.M.; Roberts, E.; Zhao, Q.; Caballero, O.L.; et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. 2013, 110, 20212–20217.
- Ahmadzadeh, M.; Rosenberg, S.A. TGF-beta 1 attenuates the acquisition and expression of effector function by tumor anti-gen-specific human memory CD8 T cells. J. Immunol. 2005, 174, 5215–5223.
- Thomas, D.A.; Massagué, J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 2005, 8, 369–380.
- Broderick, L.; Bankert, R.B. Membrane-associated TGF-beta1 inhibits human memory T cell signaling in malignant and nonmalignant inflammatory microenvironments. J. Immunol. 2006, 177, 3082–3088.
- Obermajer, N.; Muthuswamy, R.; Odunsi, K.O.; Edwards, R.P.; Kalinski, P. PGE2-Induced CXCL12 Production and CXCR4 Expression Controls the Accumulation of Human MDSCs in Ovarian Cancer Environment. Cancer Res. 2011, 71, 7463–7470.
- Deng, Y.; Cheng, J.; Fu, B.; Liu, W.; Chen, G.; Zhang, Q.; Yang, Y. Hepatic carcinoma-associated fibroblasts enhance immune suppression by facilitating the generation of myeloid-derived suppressor cells. Oncogene 2017, 36, 1090–1101.
- Gunderson, A.J.; Yamazaki, T.; Mccarty, K.; Phillips, M.; Alice, A.; Bambina, S.; Zebertavage, L.; Friedman, D.; Cottam, B.; Newell, P.; et al. Blockade of fibroblast activation protein in combination with radiation treatment in murine models of pancreatic adenocarcinoma. PLoS ONE 2019, 14, e0211117.
- Chomarat, P.; Banchereau, J.; Davoust, J.; Palucka, A.K. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat. Immunol. 2000, 1, 510–514.
- Gok Yavuz, B.; Gunaydin, G.; Gedik, M.E.; Kosemehmetoglu, K.; Karakoc, D.; Ozgur, F.; Guc, D. Cancer associated fibroblasts sculpt tumour microenvironment by recruiting monocytes and inducing immunosuppressive PD-1+ TAMs. Sci. Rep. 2019, 9, 3115–3172.
- Zhang, R.; Qi, F.; Zhao, F.; Li, G.; Shao, S.; Zhang, X.; Yuan, L.; Feng, Y. Cancer-associated fibroblasts enhance tumor-associated macrophages enrichment and suppress NK cells function in colorectal cancer. Cell Death Dis. 2019, 10, 1–14.
- Liao, D.; Luo, Y.; Markowitz, D.; Xiang, R.; Reisfeld, R.A. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS ONE 2009, 4, e7965.
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.-J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886.
- Donatelli, S.S.; Zhou, J.-M.; Gilvary, D.L.; Eksioglu, E.A.; Chen, X.; Cress, W.D.; Haura, E.B.; Schabath, M.B.; Coppola, D.; Wei, S.; et al. TGF-β-inducible microRNA-183 silences tumor-associated natural killer cells. Proc. Natl. Acad. Sci. USA 2014, 111, 4203–4208.
- Rabinovich, G.A.; Toscano, M.A. Turning “sweet” on immunity: Galectin-glycan interactions in immune tolerance and in-flammation. Nat. Rev. Immunol. 2009, 9, 338–352.
- Salmon, H.; Franciszkiewicz, K.; Damotte, D.; Dieu-Nosjean, M.-C.; Validire, P.; Trautmann, A.; Mami-Chouaib, F.; Donnadieu, E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Investig. 2012, 122, 899–910.
- Provenzano, P.; Cuevas, C.; Chang, A.; Goel, V.K.; Von Hoff, D.D.; Hingorani, S.R. Enzymatic Targeting of the Stroma Ablates Physical Barriers to Treatment of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2012, 21, 418–429.
- Ene–Obong, A.; Clear, A.J.; Watt, J.; Wang, J.; Fatah, R.; Riches, J.C.; Marshall, J.F.; Chin–Aleong, J.; Chelala, C.; Gribben, J.G.; et al. Activated Pancreatic Stellate Cells Sequester CD8+ T Cells to Reduce Their Infiltration of the Juxtatumoral Compartment of Pancreatic Ductal Adenocarcinoma. Gastroenterology 2013, 145, 1121–1132.
- Hauzenberger, D.; Olivier, P.; Gundersen, D.; Rüegg, C. Tenascin-C inhibits beta1 integrin-dependent T lymphocyte adhesion to fibronectin through the binding of its fnIII 1-5 repeats to fibronectin. Eur. J. Immunol. 1999, 29, 1435–1447.
- Armant, M.; Avice, M.N.; Hermann, P.; Rubio, M.; Kiniwa, M.; Delespesse, G.; Sarfati, M. CD47 ligation selectively down-regulates human interleukin 12 production. J. Exp. Med. 1999, 190, 1175–1182.
- Nazareth, M.R.; Broderick, L.; Simpson-Abelson, M.R.; Kelleher, R.J.; Yokota, S.J.; Bankert, R.B. Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. J. Immunol. 2007, 178, 5552–5562.
- MacGregor, H.L.; Sayad, A.; Elia, A.; Wang, B.X.; Katz, S.R.; Shaw, P.A.; Clarke, B.A.; Crome, S.Q.; Robert-Tissot, C.; Bernardini, M.Q.; et al. High expression of B7-H3 on stromal cells defines tumor and stromal compart-ments in epithelial ovarian cancer and is associated with limited immune activation. J. Immun. Cancer 2019, 7, 357.
- Castellanos, J.R.; Purvis, I.; Labak, C.M.; Guda, M.R.; Tsung, A.J.; Velpula, K.K.; Asuthkar, S. B7-H3 role in the immune landscape of cancer. Am. J. Clin. Exp. Immunol. 2017, 6, 66–75.
- Tan, W.; Zhang, W.; Strasner, A.; Grivennikov, S.; Cheng, J.Q.; Hoffman, R.M.; Karin, M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL–RANK signalling. Nature 2011, 470, 548–553.
- Li, T.; Yang, Y.; Hua, X.; Wang, G.; Liu, W.; Jia, C.; Tai, Y.; Zhang, Q.; Chen, G. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 2012, 318, 154–161.
- Balsamo, M.; Scordamaglia, F.; Pietra, G.; Manzini, C.; Cantoni, C.; Boitano, M.; Queirolo, P.; Vermi, W.; Facchetti, F.; Moretta, A.; et al. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 20847–20852.
- Li, T.; Yi, S.; Liu, W.; Jia, C.; Wang, G.; Hua, X.; Tai, Y.; Zhang, Q.; Chen, G. Colorectal carcinoma-derived fibroblasts modulate natural killer cell phenotype and antitumor cytotoxicity. Med. Oncol. 2013, 30, 663–667.
- Lakins, M.A.; Ghorani, E.; Munir, H.; Martins, C.P.; Shields, J.D. Cancer-associated fibroblasts induce antigen-specific deletion of CD8+T Cells to protect tumour cells. Nat. Comms 2018, 9, 948.
- Arwert, E.N.; Milford, E.L.; Rullan, A.; Derzsi, S.; Hooper, S.; Kato, T.; Mansfield, D.; Melcher, A.; Harrington, K.J.; Sahai, E. Author Correction: STING and IRF3 in stromal fibroblasts enable sensing of genomic stress in cancer cells to undermine oncolytic viral therapy. Nat. Cell Biol. 2020, 22, 908.
- Barker, H.E.; Paget, J.T.E.; Khan, A.; Harrington, K. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425.
- Hirata, E.; Sahai, E. Tumor Microenvironment and Differential Responses to Therapy. Cold Spring Harb. Perspect. Med. 2017, 7, a026781.
- Linares, J.; Marín-Jiménez, J.A.; Badia-Ramentol, J.; Calon, A. Determinants and Functions of CAFs Secretome During Cancer Progression and Therapy. Front. Cell Dev. Biol. 2020, 8, 621070.
- Rodier, F.; Coppé, J.-P.; Patil, C.K.; Hoeijmakers, W.A.M.; Muñoz, D.P.; Raza, S.R.; Freund, A.; Campeau, E.; Davalos, A.R.; Campisi, J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009, 11, 973–979.
- Saleh, T.; Tyutynuk-Massey, L.; Cudjoe, E.; Idowu, M.O.; Landry, J.W.; Gewirtz, D.A. Non-Cell Autonomous Effects of the Senescence-Associated Secretory Phenotype in Cancer Therapy. Front. Oncol. 2018, 8, 164.
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.-W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990.
- Rao, S.G.; Jackson, J.G. SASP: Tumor Suppressor or Promoter? Yes! Trends Cancer 2016, 2, 676–687.
- Wang, C.; Xi, W.; Jiang, J.; Ji, J.; Yu, Y.; Zhu, Z.; Zhang, J. Metronomic chemotherapy remodel cancer-associated fibroblasts to decrease chemoresistance of gastric cancer in nude mice. Oncol. Lett. 2017, 14, 7903–7909.
- Toste, P.A.; Nguyen, A.H.; Kadera, B.; Duong, M.; Wu, N.; Gawlas, I.; Tran, L.M.; Bikhchandani, M.; Li, L.; Patel, S.G.; et al. Chemotherapy-Induced Inflammatory Gene Signature and Protumorigenic Phenotype in Pancreatic CAFs via Stress-Associated MAPK. Mol. Cancer Res. 2016, 14, 437–447.
- Sun, Y.; Campisi, J.; Higano, C.; Beer, T.M.; Porter, P.; Coleman, I.; True, L.; Nelson, P.S. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat. Med. 2012, 18, 1359–1368.
- Tao, L.; Huang, G.; Wang, R.; Pan, Y.; He, Z.; Chu, X.; Song, H.; Chen, L. Cancer-associated fibroblasts treated with cisplatin facilitates chemoresistance of lung adenocarcinoma through IL-11/IL-11R/STAT3 signaling pathway. Sci. Rep. 2016, 6, 38408.
- Brown, E.; McKee, T.; diTomaso, E.; Pluen, A.; Seed, B.; Boucher, Y.; Jain, R.K. Dynamic imaging of collagen and its modula-tion in tumors in vivo using second-harmonic generation. Nat. Med. 2003, 9, 796–800.
- Richards, K.E.; Zeleniak, A.E.; Fishel, M.; Wu, J.; Littlepage, L.E.; Hill, R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene 2017, 36, 1770–1778.
- Zhang, H.; Deng, T.; Liu, R.; Ning, T.; Yang, H.; Liu, D.; Zhang, Q.; Lin, D.; Ge, S.; Bai, M.; et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol. Cancer 2020, 19, 1–17.
- Yarnold, J.; Brotons, M.-C.V. Pathogenetic mechanisms in radiation fibrosis. Radiother. Oncol. 2010, 97, 149–161.
- Verset, L.; Tommelein, J.; Lopez, X.M.; Decaestecker, C.; Boterberg, T.; De Vlieghere, E.; Salmon, I.; Mareel, M.; Bracke, M.; De Wever, O.; et al. Impact of neoadjuvant therapy on cancer-associated fibroblasts in rectal cancer. Radiother. Oncol. 2015, 116, 449–454.
- Matsuoka, Y.; Yoshida, R.; Nakayama, H.; Nagata, M.; Hirosue, A.; Tanaka, T.; Kawahara, K.; Nakagawa, Y.; Sakata, J.; Arita, H.; et al. The tumour stromal features are associated with resistance to 5-FU-based chemoradiotherapy and a poor prognosis in patients with oral squamous cell carcinoma. APMIS 2014, 123, 205–214.
- Farmer, P.; Bonnefoi, H.; Anderle, P.; A Cameron, D.; Wirapati, P.; Becette, V.; André, S.; Piccart-Gebhart, M.; Campone, M.; Brain, E.; et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat. Med. 2009, 15, 68–74.
- Knops, A.M.; South, A.; Rodeck, U.; Martinez-Outschoorn, U.; Harshyne, L.A.; Johnson, J.; Luginbuhl, A.J.; Curry, J.M. Cancer-Associated Fibroblast Density, Prognostic Characteristics, and Recurrence in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Front. Oncol. 2020, 10, 565306.
- Cordes, N. Integrin-mediated cell–matrix interactions for prosurvival and antiapoptotic signaling after genotoxic injury. Cancer Lett. 2006, 242, 11–19.
- Goel, H.L.; Sayeed, A.; Breen, M.; Zarif, M.J.; Garlick, D.S.; Leav, I.; Davis, R.J.; Fitzgerald, T.J.; Morrione, A.; Hsieh, C.C.; et al. β1 integrins mediate resistance to ionizing radiation in vivo by inhibit-ing c-Jun amino terminal kinase 1. J. Cellul. Physiol. 2013, 228, 1601–1609.
- Cordes, N.; Seidler, J.; Durzok, R.; Geinitz, H.; Brakebusch, C. beta1-integrin-mediated signaling essentially contributes to cell survival after radiation-induced genotoxic injury. Oncogene 2006, 25, 1378–1390.
- Tommelein, J.; De Vlieghere, E.; Verset, L.; Melsens, E.; Leenders, J.; Descamps, B.; Debucquoy, A.; Vanhove, C.; Pauwels, P.; Gespach, C.P.; et al. Radiotherapy-Activated Cancer-Associated Fibroblasts Promote Tumor Progression through Paracrine IGF1R Activation. Cancer Res. 2017, 78, 659–670.
- Garvey, C.M.; Lau, R.; Sanchez, A.; Sun, R.X.; Fong, E.J.; Doche, M.E.; Chen, O.; Jusuf, A.; Lenz, H.-J.; Larson, B.; et al. Anti-EGFR Therapy Induces EGF Secretion by Cancer-Associated Fibroblasts to Confer Colorectal Cancer Chemoresistance. Cancers 2020, 12, 1393.
- Madden, E.C.; Gorman, A.M.; Logue, S.E.; Samali, A. Tumour Cell Secretome in Chemoresistance and Tumour Recurrence. Trends Cancer 2020, 6, 489–505.
- Maia, A.; Gu, Z.; Koch, A.; Berdiel-Acer, M.; Will, R.; Schlesner, M.; Wiemann, S. IFNβ1 secreted by breast cancer cells un-dergoing chemotherapy reprograms stromal fibroblasts to support tumour growth after treatment. Mol. Oncol. 2021, 15, 1308–1329.
- Mackenzie, K.J.; Carroll, P.; Martin, C.-A.; Murina, O.; Fluteau, A.; Simpson, D.J.; Olova, N.; Sutcliffe, H.; Rainger, J.K. cGAS surveil-lance of micronuclei links genome instability to innate immunity. Nature 2017, 548, 461–465.
- Yang, H.; Wang, H.; Ren, J.; Chen, Q.; Chen, Z.J. cGAS is essential for cellular senescence. Proc. Natl. Acad. Sci. USA 2017, 114, E4612–E4620.
- Glück, S.; Guey, B.; Gulen, M.F.; Wolter, K.; Kang, T.-W.; Schmacke, N.A.; Bridgeman, A.; Rehwinkel, J.; Zender, L.; Ablasser, A. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat. Cell Biol. 2017, 19, 1061–1070.
- Kioi, M.; Vogel, O.H.; Schultz, G.; Hoffman, R.M.; Harsh, G.R.; Brown, J.M. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J. Clin. Investig. 2010, 120, 694–705.
- Rey, S.; Semenza, G.L. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc. Res. 2010, 86, 236–242.
- Fedorenko, I.V.; Wargo, J.A.; Flaherty, K.T.; Messina, J.L.; Smalley, K.S. BRAF Inhibition Generates a Host–Tumor Niche that Mediates Therapeutic Escape. J. Investig. Dermatol. 2015, 135, 3115–3124.
- Pennisi, A.; Li, X.; Gaddy, D.; Akel, N.; Aziz, N.; Yaccoby, S. Inhibitors of Fibroblast Activation Protein (FAP) Inhibit Primary Myeloma Growth and Osteoclastogenesis Ex Vivo and In Vivo. Blood 2007, 110, 813.
- Jackson, K.W.; Christiansen, V.J.; Yadav, V.R.; Silasi-Mansat, R.; Lupu, F.; Awasthi, V.; Zhang, R.R.; McKee, P.A. Suppression of tumor growth in mice by rationally designed pseudopeptide inhibitors of fibroblast activation protein and prolyl oligopeptidase. Neoplasia 2015, 17, 43–54.
- Adams, S.; Miller, G.T.; Jesson, M.I.; Watanabe, T.; Jones, B.; Wallner, B.P. PT-100, a small molecule dipeptidyl peptidase inhibitor, has potent antitumor effects and augments antibody-mediated cytotoxicity via a novel immune mechanism. Cancer Res 2004, 64, 5471–5480.
- Walsh, M.P.; Duncan, B.; Larabee, S.; Krauss, A.; Davis, J.P.E.; Cui, Y.; Kim, S.Y.; Guimond, M.; Bachovchin, W.; Fry, T.J. Val-boroPro accelerates T cell priming via modulation of dendritic cell trafficking resulting in complete regression of es-tablished murine tumors. PLoS ONE 2013, 8, e58860.
- Ostermann, E.; Garin-Chesa, P.; Heider, K.H.; Kalat, M.; Lamche, H.; Puri, C.; Kerjaschki, D.; Rettig, W.J.; Adolf, G.R. Effec-tive immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts. Clin. Cancer Res. 2008, 14, 4584–4592.
- Loeffler, M.; Krüger, J.A.; Niethammer, A.G.; Reisfeld, R.A. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J. Clin. Investig. 2006, 116, 1955–1962.
- Welt, S.; Divgi, C.R.; Scott, A.M.; Garin-Chesa, P.; Finn, R.D.; Graham, M.; A Carswell, E.; Cohen, A.; Larson, S.M.; Old, L.J.; et al. Antibody targeting in metastatic colon cancer: A phase I study of monoclonal antibody F19 against a cell-surface protein of reactive tumor stromal fibroblasts. J. Clin. Oncol. 1994, 12, 1193–1203.
- Scott, A.M.; Wiseman, G.; Welt, S.; Adjei, A.; Lee, F.-T.; Hopkins, W.; Divgi, C.R.; Hanson, L.H.; Mitchell, P.; Gansen, D.N.; et al. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast ac-tivation protein-positive cancer. Clin. Cancer Res. 2003, 9, 1639–1647.
- Nemunaitis, J.; Vukelja, S.J.; Richards, D.; Cunningham, C.; Senzer, N.; Nugent, J.; Duncan, H.; Jones, B.; Haltom, E.; Uprich-ard, M.J. Phase I trial of PT-100 (PT-100), a cytokine-inducing small molecule, following chemotherapy for solid tumor ma-lignancy. Cancer Invest. 2006, 24, 553–561.
- Narra, K.; Mullins, S.R.; Lee, H.-O.; Strzemkowski-Brun, B.; Magalong, K.; Christiansen, V.J.; McKee, P.A.; Egleston, B.; Cohen, S.J.; Weiner, L.M.; et al. Phase II trial of single agent Val-boroPro (Talabostat) inhibiting Fi-broblast Activation Protein in patients with metastatic colorectal cancer. Cancer Biol. Ther. 2007, 6, 1691–1699.
- Eager, R.; Cunningham, C.; Senzer, N.; Richards, D.; Raju, R.; Jones, B.; Uprichard, M.; Nemunaitis, J. Phase II Trial of Talabostat and Docetaxel in Advanced Non-small Cell Lung Cancer. Clin. Oncol. 2009, 21, 464–472.
- Eager, R.M.; Cunningham, C.C.; Senzer, N.N.; Stephenson, J.; Anthony, S.P.; O’Day, S.J.; Frenette, G.; Pavlick, A.C.; Jones, B.; Uprichard, M.; et al. Phase II assessment of talabostat and cisplatin in second-line stage IV melanoma. BMC Cancer 2009, 9, 263.
- Herter, S.; Morra, L.; Schlenker, R.; Sulcova, J.; Fahrni, L.; Waldhauer, I.; Lehmann, S.; Reisländer, T.; Agarkova, I.; Kelm, J.M.; et al. A novel three-dimensional heterotypic spheroid model for the assessment of the activity of cancer immunotherapy agents. Cancer Immunol. Immunother. CII 2017, 66, 129–140.
- Kermer, V.; Baum, V.; Hornig, N.; Kontermann, R.E.; Müller, D. An Antibody Fusion Protein for Cancer Immunotherapy Mimicking IL-15 trans-Presentation at the Tumor Site. Mol. Cancer Ther. 2012, 11, 1279–1288.
- Hornig, N.; Kermer, V.; Frey, K.; Diebolder, P.; Kontermann, R.E.; Müller, D. Combination of a Bispecific Antibody and Costimulatory Antibody-Ligand Fusion Proteins for Targeted Cancer Immunotherapy. J. Immunother. 2012, 35, 418–429.
- Sum, E.; Rapp, M.; Fröbel, P.; Le Clech, M.; Dürr, H.; Giusti, A.M.; Perro, M.; Speziale, D.; Kunz, L.; Menietti, E.; et al. Fibroblast Activation Protein α-Targeted CD40 Agonism Abrogates Systemic Toxicity and Enables Administration of High Doses to Induce Effective Antitumor Immunity. Clin. Cancer Res. 2021.
- Fang, J.; Xiao, L.; Joo, K.-I.; Liu, Y.; Zhang, C.; Liu, S.; Conti, P.S.; Li, Z.; Wang, P. A potent immunotoxin targeting fibroblast activation protein for treatment of breast cancer in mice. Int. J. Cancer 2016, 138, 1013–1023.
- Fabre, M.; Ferrer, C.; Domínguez-Hormaetxe, S.; Bockorny, B.; Murias, L.; Seifert, O.; Eisler, S.A.; Kontermann, R.E.; Pfizenmaier, K.; Lee, S.Y.; et al. OMTX705, a Novel FAP-Targeting ADC Demonstrates Activity in Chemotherapy and Pembrolizumab-Resistant Solid Tumor Models. Clin. Cancer Res. 2020, 26, 3420–3430.
- Kakarla, S.; Chow, K.K.H.; Mata, M.; Shaffer, D.R.; Song, X.-T.; Wu, M.-F.; Liu, H.; Wang, L.L.; Rowley, D.R.; Pfizenmaier, K.; et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol. Ther. 2013, 21, 1611–1620.
- Wang, L.-C.S.; Lo, A.; Scholler, J.; Sun, J.; Majumdar, R.S.; Kapoor, V.; Antzis, M.; Cotner, C.E.; Johnson, L.A.; Durham, A.C.; et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol. Res. 2014, 2, 154–166.
- Park, C.C.; Zhang, H.; Pallavicini, M.; Gray, J.W.; Baehner, F.; Park, C.J.; Bissell, M.J. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006, 66, 1526–1535.
- Gerber, D.E.; Camidge, D.R.; Morgensztern, D.; Cetnar, J.; Kelly, R.J.; Ramalingam, S.S.; Spigel, D.R.; Jeong, W.; Scaglioni, P.P.; Zhang, S.; et al. Phase 2 study of the focal adhesion kinase inhibitor defactinib (VS-6063) in previously treated advanced KRAS mutant non-small cell lung cancer. Lung Cancer 2020, 139, 60–67.
- Ebbinghaus, C.; Scheuermann, J.; Neri, D.; Elia, G. Diagnostic and therapeutic applications of recombinant antibodies: Targeting the extra-domain B of fibronectin, a marker of tumor angiogenesis. Curr Pharm Des 2004, 10, 1537–1549.
- O’Connell, J.T.; Sugimoto, H.; Cooke, V.G.; MacDonald, B.A.; Mehta, A.I.; LeBleu, V.S.; Dewar, R.; Rocha, R.M.; Brentani, R.R.; Resnick, M.B.; et al. VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc. Natl. Acad. Sci. USA 2011, 108, 16002–16007.
- Chauhan, V.P.; Martin, J.D.; Liu, H.; Lacorre, D.A.; Jain, S.R.; Kozin, S.V.; Stylianopoulos, T.; Mousa, A.S.; Han, X.; Adstamongkonkul, P.; et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Comms. 2013, 4, 2516–2611.
- Jacobetz, M.A.; Chan, D.S.; Neesse, A.; Bapiro, T.E.; Cook, N.; Frese, K.K.; Feig, C.; Nakagawa, T.; Caldwell, M.E.; Zecchini, H.I.; et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2013, 62, 112–120.
- Hingorani, S.R.; Harris, W.P.; Beck, J.T.; Berdov, B.A.; Wagner, S.A.; Pshevlotsky, E.M.; Tjulandin, S.A.; Gladkov, O.A.; Holcombe, R.F.; Korn, R.; et al. Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 2016, 22, 2848–2854.
- Hingorani, S.R.; Zheng, L.; Bullock, A.J.; Seery, T.E.; Harris, W.P.; Sigal, D.S.; Braiteh, F.; Ritch, P.S.; Zalupski, M.M.; Bahary, N.; et al. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J. Clin. Oncol. 2018, 36, 359–366.
- Murphy, J.E.; Wo, J.Y.; Ryan, D.P.; Clark, J.W.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Ly, L.; Baglini, C.V.; Blaszkowsky, L.S.; et al. Total Neoadjuvant Therapy With FOLFIRINOX in Combination With Losartan Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancer: A Phase 2 Clinical Trial. JAMA Oncol 2019, 5, 1020–1027.
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801.
- Vandenbroucke, R.E.; Libert, C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug. Discov. 2014, 13, 904–927.
- Caruana, I.; Savoldo, B.; Hoyos, V.; Weber, G.; Liu, H.; Kim, E.S.; Ittmann, M.M.; Marchetti, D.; Dotti, G. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med. 2015, 21, 524–529.
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248.
- Giannoni, E.; Bianchini, F.; Masieri, L.; Serni, S.; Torre, E.; Calorini, L.; Chiarugi, P. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010, 70, 6945–6956.
- Karakasheva, T.A.; Lin, E.W.; Tang, Q.; Qiao, E.; Waldron, T.J.; Soni, M.; Klein-Szanto, A.J.; Sahu, V.; Basu, D.; Ohashi, S.; et al. IL-6 Mediates Cross-Talk between Tumor Cells and Activated Fibroblasts in the Tumor Microenvironment. Cancer Res 2018, 78, 4957–4970.
- Bournazou, E.; Bromberg, J. Targeting the tumor microenvironment: JAK-STAT3 signaling. JAKSTAT 2013, 2, e23828.
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809.
- Fizazi, K.; De Bono, J.S.; Flechon, A.; Heidenreich, A.; Voog, E.; Davis, N.B.; Qi, M.; Bandekar, R.; Vermeulen, J.T.; Cornfeld, M.; et al. Randomised phase II study of siltuximab (CNTO 328), an anti-IL-6 monoclonal antibody, in combination with mitoxantrone/prednisone versus mitoxantrone/prednisone alone in metastatic castration-resistant prostate cancer. Eur. J. Cancer 2012, 48, 85–93.
- McLeod, R.; Kumar, R.; Papadatos-Pastos, D.; Mateo, J.; Brown, J.S.; Garces, A.H.I.; Ruddle, R.; Decordova, S.; Jueliger, S.; Ferraldeschi, R.; et al. First-in-Human Study of AT13148, a Dual ROCK-AKT Inhibitor in Patients with Solid Tumors. Clin. Cancer Res. 2020, 26, 4777–4784.
- Reilley, M.J.; McCoon, P.; Cook, C.; Lyne, P.; Kurzrock, R.; Kim, Y.; Woessner, R.; Younes, A.; Nemunaitis, J.; Fowler, N.; et al. STAT3 antisense oligonucleotide AZD9150 in a subset of patients with heavily pretreated lymphoma: Results of a phase 1b trial. J. Immun. Cancer 2018, 6, 119.
- Burger, J.A.; Peled, A. CXCR4 antagonists: Targeting the microenvironment in leukemia and other cancers. Leukemia 2009, 23, 43–52.
- Eckert, F.; Schilbach, K.; Klumpp, L.; Bardoscia, L.; Sezgin, E.C.; Schwab, M.; Zips, D.; Huber, S.M. Potential Role of CXCR4 Targeting in the Context of Radiotherapy and Immunotherapy of Cancer. Front. Immunol. 2018, 9, 3018.
- Ghobrial, I.M.; Liu, C.-J.; Zavidij, O.; Azab, A.K.; Baz, R.; Laubach, J.P.; Mishima, Y.; Armand, P.; Munshi, N.C.; Basile, F.; et al. Phase I/II trial of the CXCR4 inhibitor plerixafor in combination with bortezomib as a chemosensitization strategy in relapsed/refractory multiple myeloma. Am. J. Hematol. 2019, 94, 1244–1253.
- Lee, E.Q.; Duda, D.G.; Muzikansky, A.; Gerstner, E.R.; Kuhn, J.G.; Reardon, D.A.; Nayak, L.; Norden, A.D.; Doherty, L.; LaFrankie, D.; et al. Phase I and Biomarker Study of Plerixafor and Bevacizumab in Recurrent High-Grade Glioma. Clin. Cancer Res. 2018, 24, 4643–4649.
- Rimkus, T.K.; Carpenter, R.L.; Qasem, S.; Chan, M.; Lo, H.-W. Targeting the Sonic Hedgehog Signaling Pathway: Review of Smoothened and GLI Inhibitors. Cancers 2016, 8, 22.
- Carpenter, R.L.; Ray, H. Safety and Tolerability of Sonic Hedgehog Pathway Inhibitors in Cancer. Drug Saf. 2019, 42, 263–279.
- Biswas, S.; Guix, M.; Rinehart, C.; Dugger, T.C.; Chytil, A.; Moses, H.L.; Freeman, M.L.; Arteaga, C.L. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J. Clin. Investig. 2007, 117, 1305–1313.
- Bouquet, F.; Pal, A.; Pilones, K.A.; Demaria, S.; Hann, B.; Akhurst, R.J.; Babb, J.S.; Lonning, S.M.; DeWyngaert, J.K.; Formenti, S.C.; et al. TGFβ1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin. Cancer Res. 2011, 17, 6754–6765.
- Neuzillet, C.; Tijeras-Raballand, A.; Cohen, R.; Cros, J.; Faivre, S.; Raymond, E.; de Gramont, A. Targeting the TGFβ pathway for cancer therapy. Pharmacol. Ther. 2015, 147, 22–31.
More
