Functional hypothalamic amenorrhea is the most common cause of secondary amenorrhea in women of childbearing age. It is a reversible disorder caused by stress related to weight loss, excessive exercise and/or traumatic mental experiences. The basis of functional hypothalamic amenorrhea is hormonal, based on impaired pulsatile GnRH secretion in the hypothalamus, then decreased secretion of gonadotropins, and, consequently, impaired hormonal function of the ovaries. This disorder leads to hypoestrogenism, manifested by a disturbance of the menstrual cycle in the form of amenorrhea, leading to anovulation. Prolonged state of hypoestrogenism can be very detrimental to general health, leading to many harmful short- and long-term consequences.
1. Introduction
The origin of FHA is believed to rely on impaired pulsatile gonadotropin releasing hormone (GnRH) secretion. Abnormal GnRH secretion causes a reduction in the pulses of both the luteinizing hormone (LH) and follicle stimulating hormone (FSH). As a consequence, there is no increase in LH secretion in the middle of the menstrual cycle, a lack of proper follicular development, successive anovulation and a low serum estradiol (E2) concentration [1][3]. It is said that FHA may have a genetic predisposition, such as heterozygosity for congenital hypogonadotropic hypogonadism [2][4]. Hypoestrogenism that results from the inhibition of the hypothalamus-pituitary- ovarian (HPO) axis has profound effects on many systems throughout the body, including reproductive, cardiac, skeletal and psychological. That is why it is so important to apply appropriate treatment as soon as possible to prevent numerous short- and long-term health consequences [3][5].
Initial management should focus on treating the underlying cause, that is, trying to gain weight, limiting exercise, or avoiding emotional stress. In situations where such treatment is not effective, hormone replacement therapy should be used to maintain normal estrogen levels, or if the patient wishes to become pregnant, treatment focused on ovulation induction or in vitro fertilization (IVF) [4][6].
2. Patophysiology
The mechanisms responsible for the development of FHA are not fully understood and still seem unclear. Certainly, numerous neurotransmitters and neurosteroids play an important role in the regulation of the hypothalamic-pituitary-ovarian axis, therefore they most likely also play a role in the pathophysiology of FHA. The most important seem to be kisspeptin, leptin, beta-endorphin, neuropeptide Y (NPY), ghrelin, and corticotropin releasing hormone (CRH). Kisspeptin, which is a product of the KiSS-1 gene and its G. The receptor coupled to the GPR54 protein plays a key role in regulation of reproductive functions. Kisspeptin/GPR54 the system stimulates the hypothalamic-pituitary-ovary axis, but can also directly stimulate the secretion of GnRH from the hypothalamus
[5][7].
The 2017 Endocrine Society Clinical Practice Guidelines on FHA stress that FHA is a form of chronic ovulation caused not by an organic cause, but by various types of stress, resulting from weight loss, excessive physical exertion or traumatic psychological experiences
[6][2].
First of all, the influence of different kinds of stress may negatively affect reproduction along the entire hypothalamic-pituitary-ovarian axis. FHA is characterized by a disturbance in pulsatile GnRH secretion, leading to a reduction in the amplitude and/or frequency of gonadotropin pulses and anovulation. Varying neuroendocrine patterns of LH secretion can be observed in women with FHA. Regarding serum FSH levels, low or normal levels are reported, but often above LH levels
[7][8]. Low energy availability due to increased caloric expenditure and/or insufficient caloric intake can have a negative inhibitory effect on the HPO axis, redirecting energy from reproductive processes to more vital systems for survival
[8][9].
Additionally, the effect on the axis of growth hormone and insulin-like growth factor 1 (GH- IGF-1) in a situation of energy deficiency, either due to reduced caloric consumption, excessive energy expenditure or both, is a reduced IGF-1 level, despite an increase in GH levels, as a result of a nutritionally acquired resistance to GH
[9][10].
Another hormone axis that suffers from FHA is the adrenal axis. Strong stressful situations can interact by activating the hypothalamus-pituitary-adrenal (HPA) axis
[10][11]. This activation is associated with increased secretion of the hypothalamus of the corticotropin releasing hormone (CRH), corticotropin (ACTH) and adrenal cortisol. CRH works by inhibiting the frequency of GnRH pulses, while cortisol inhibits reproductive function at the hypothalamus, pituitary and uterine levels. Therefore, in women diagnosed with FHA, we most often observe higher basal cortisol levels and diminished ACTH and cortisol responses to CRH stimulation. This is most likely secondary to the negative feedback associated with hypercortisolemia and elevated ACTH levels
[11][12].
An interesting point is that there is considerable variability in the degree of weight loss or physical exertion necessary to induce amenorrhea. This provides grounds for considering the genetic predisposition of individual women to develop FHA. Many gene mutations such as (KAL1, FGFR1, PROKR2, GNRHR) have been identified in patients with congenital GnRH deficiency. Rare variants exist in the genes associated with idiopathic hypogonadotrophic hypogonadism in FHA patients. This suggests that individual mutations may contribute to a greater or lesser susceptibility of women to functional changes, such as exercise, stress or weight loss. It follows from this that depending on individual mutations and genetic conditions, sensitivity to the inhibition of the hypothalamic—pituitary—gonadal axis by such stressors varies substantially. However, it is not known exactly whether this susceptibility reflects a genetic predisposition to hypothalamic amenorrhea
[2][4].
3. Diagnosis of FHA
The diagnosis of FHA is associated with amenorrhea associated with low serum levels of gonadotropins and hypoestrogenism. Additionally, it is essential to find the cause of these symptoms, i.e., a stress factor such as low body weight, exercise or emotional stress. FHA diagnosis is made by exclusion, and it is very important to rule out other causes of amenorrhea.
The assessment of a patient with FHA should focus on a thorough medical history and gynecological examination. In particular, the interview must include gathering information on the relationship between disturbances in the menstrual cycle and weight loss, overexertion or experienced work- or study-related stress and personal stress, in addition to psychiatric disorders such as anxiety and depression.
The next step in the appropriate evaluation of patients with FHA is biochemical testing. In addition to excluding pregnancy and testing for hCG levels, thyroid-stimulating hormone (TSH), free thyroxine (T4), prolactin, luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol (E2), and antiMullerian hormone (AMH) should be measured. Additionally, complete blood count, electrolytes, glucose, bicarbonate, blood urea nitrogen, creatinine, liver panel, and (when appropriate) sedimentation rate and/or C-reactive protein levels should be performed.
Another essential test that should not be forgotten is bone densitometry.
Due to the possibility of mood disorders in patients with FHA, tests to assess anxiety and mood disorders should be performed.
Standard fertility assessments should be performed by all patients with FHA.
4. Consequences of FHA
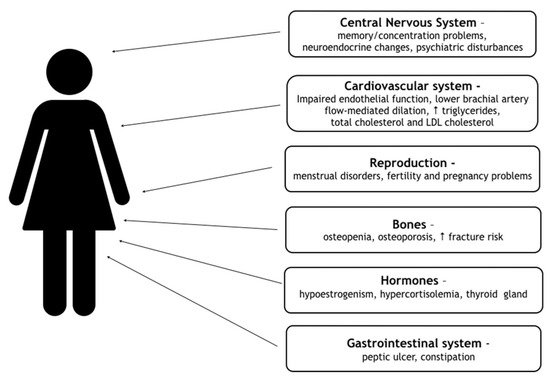
Women with FHA are exposed to numerous health consequences of hypoestrogenism. They have a greater risk of developing complications in the reproductive, cardiovascular, skeletal system and psychiatric comorbidities, including anxiety and mood disorders. All consequences are contained in Figure 1 [12][19].
Figure 1.
Consequences of FHA. (↑-increased)
5. Treatment
The most appropriate and effective treatment for FHA is that of treating the underlying cause of hypogonadotropic hypogonadism. It includes providing the right amount of energy in the form of sufficient calories, stopping excessive sports or reducing stress, or the ability to deal with it. Only when treatment is effective is it possible to prevent the many complications of FHA. Very often, anxiety and mood disorders coexist with FHA, therefore the help of a psychologist and psychiatrist should be taken into account. Hospital treatment is required in the event of cardiac arrhythmias, most often bradycardia, water and electrolyte disturbances, or in cases of extreme malnutrition associated with eating disorders.
6. Conclusions
FHA is a common disorder that most commonly affects adolescents and reproductive women. The cause of the disorder is widely understood to be stress in various forms. Disorders caused by limiting caloric consumption are observed, causing weight loss, intensive sports by the patient and the occurrence of strong stressful experiences. The diagnosis of FHA should be made as soon as possible, taking into account a thorough medical history and analysis of the patient’s history, excluding other causes of amenorrhea. This is important, because providing the patient with immediate care and introducing pharmacological treatment at the right time avoids many harmful consequences that extend far beyond the reproductive system. Treatment is best carried out in cooperation with a gynecologist, endocrinologist, psychologist/psychiatrist and a dietitian. The goal of the future is to introduce new drugs that may prove to be more effective than the hormone replacement therapy used so far in most countries.