Oxidative Strong Metal–Support Interactions (OMSI) can be defined as a phenomenon occurring in a supported metal catalyst that is triggered by oxidative (or non-reductive) conditions with the typical features resembling that of SMSI, including 1) small-molecule of CO or H2 adsorption on metal will be significantly suppressed, 2) the support would encapsulate metal particles, 3) electron transfer from metal to the support, and 4) a reversal of the above phenomena following reduction treatment.
- oxidative strong metal–support interactions
- strong metal–support interaction
- supported metal catalyst
- metal-oxide interfaces
- charge transfer
- heterogeneous catalysis
1. Introduction
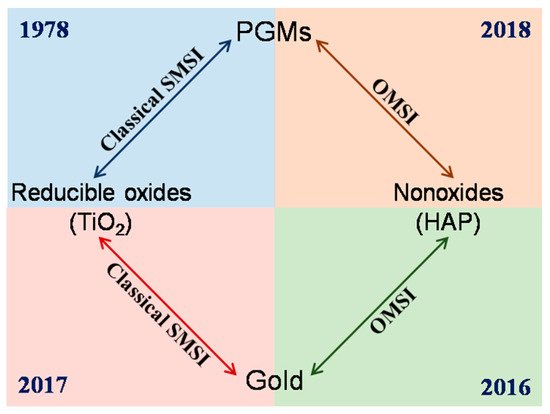
Strong metal-support interaction (SMSI) is a phenomenon discovered by Tauster et al in the late 1970s, that TiO2 supported Pt-group metals will lose their capability to adsorb small molecules (such as CO and H2) following high-temperature reduction. [1][2] SMSI between Pt-group metals and reducible oxides has continuously been studied in both catalysis application and surface science for the next three decades, and the understanding on it has progressed significantly. The classical SMSI theory thus developed, based on which the SMSI-active catalyst system was limited. Au (and IB group metals), with a relatively lower work function and surface energy respect to Pt-group metals, had thus been regarded as SMSI-inert for a long time. [3][4][5][6]
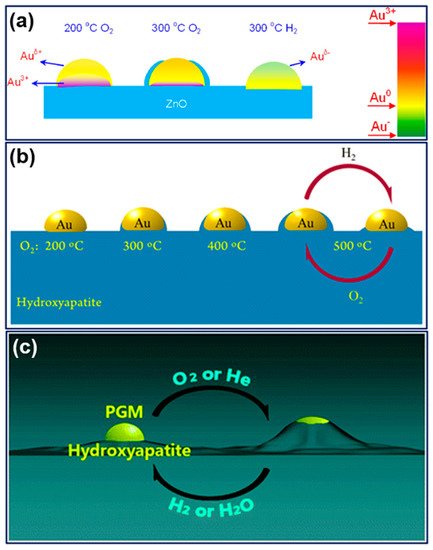
A breakthrough came from Mou’s group in 2012.[7] They found an oxygen-induced SMSI phenomenon in an Au/ZnO-nanorod catalyst, that, following oxidation under 300 °C, the Au nanoparticles will be encapsulated by ZnO accompanied by electron transfer from Au to the support, which will be reversed by hydrogen treatment. This work, for the first time, not only extended the conditions for evoking SMSI but also opened a prelude for the study of SMSI in Au-based catalysts. Only about four years later, a similar oxidative SMSI phenomenon was revealed by Tang et al. in nonoxide (hydroxyapatite and phosphate)-supported Au catalysts, expanding the territory of SMSI to the non-oxide support system.[8] Furthermore, in 2018, they confirmed that the nonoxide- and ZnO-supported Pt-group metals are also applicable for this oxygen-induced SMSI, and formally proposed the concept of “oxidative strong metal–support interactions (OMSI)” to distinguish from classical SMSI, which was triggered by reductive conditions.[9] It has been proved that controlled OMSI is effective in tuning the catalyst performances,[7][8][9][10] and a few discoveries inspired by OMSI were consecutively reported thereafter, such as uncovering the classical SMSI between Au (actually the IB group metals) and TiO2, and SMSI between Au and layered double hydroxide support.[11][12]
2. Features, and Catalyst Systems of OMSI
OMSI can be defined as a phenomenon occurring in supported metal catalyst that triggered by oxidative (or non-reductive) conditions with the typical features resemble with that of SMSI. Current OMSI systems involve ZnO-nanorod supported Au, nonoxides (hydroxyapatite and phosphate) supported Au and Pt-group metals, and ZnO supported Pt-group metals (Figure 1). The common characteristics of OMSI evoked by high-temperature oxidation (or an inert atmosphere) can be summarized as: (1) small-molecule of CO or H2 adsorption on metal will be significantly suppressed; (2) mass transport that the support would encapsulate metal particles; (3) electron transfer from metal to the support resulting in a positively charged metal species; and (4) a reversal of the above phenomena following reduction treatment. The comparison of the inducing conditions and main characteristics of OMSI and the classical SMSI was listed in Table 1.
| Classical SMSI | OMSI | |
|---|---|---|
| typical catalyst system | reducible oxide-supported Au or Pt-group metals | HAP- or ZnO-supported Au or Pt-group metals |
| inducing conditions | high-temperature reduction | high-temperature oxidation |
| suppression of adsorption | yes | yes |
| mass transport (encapsulation) | yes | yes |
| electron transfer | support to metal | metal to support |
| reversibility | yes | yes |
3. Identification and Characterization of OMSI
The abovementioned four typical features are also the criteria for the identification of OMSI occurrence, which generally needs a combination of multiple characterization methods to confirm.
3.1. Adsorption Behavior
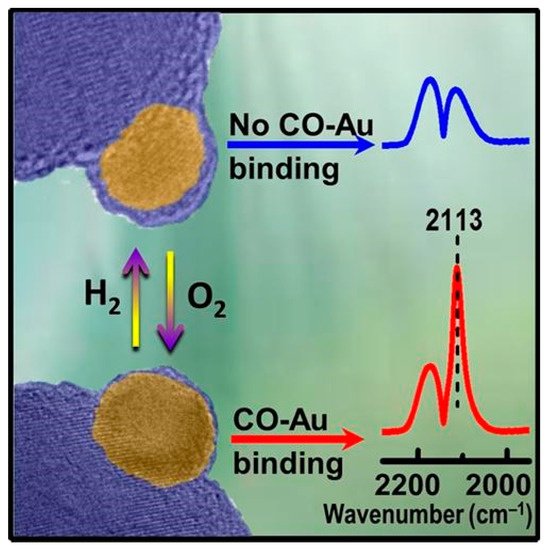
One of the most typical spectroscopic techniques is the in situ molecular probe infrared (IR) spectroscopy. Due to site-specific sensitivity in detecting the adsorption property of metal surface, it has been popularized in heterogeneous catalysis and become an indispensable approach to measure adsorption behavior of catalysts in the SMSI study.[13][7][8][9][10][11][12][14]
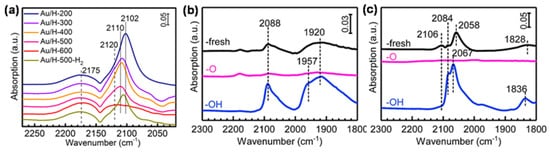
Tang et al. carried out an in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) measurement of CO adsorption on Au/HAP treated by high-temperature oxidation under different temperatures, an instructive case for the application of IR spectroscopy to the identification of OMSI.[8] As shown in Figure 2a, the bands at 2102~2110 cm−1 were ascribed to the CO adsorbed on the Au surface (CO-Au). Similar measurements were further carried out to prove the reversible suppression of chemisorption on Pd/HAP and Pt/HAP by high-temperature oxidation/reduction, confirming the occurrence of OMSI in Pt-group metal-based catalysts (Figure 2b,c).[9]
3.2. Mass Transport
Mass transport in OMSI mainly refers to the reversible encapsulation of metal by the deformed support, which generally can be intuitively observed by high resolution transmission electron microscopy (HRTEM). In 1985, Singh et al. directly observed a thin overlayer on the Rh surface through HRTEM in Rh/TiO2 where SMSI occurred, thus inferring that the overlayer inhibited the adsorption of small molecules on the catalyst.[15] Since then, the SMSI-provoked encapsulation phenomenon has been found in various catalysts, becoming an essential basis for identifying the occurrence of SMSI, and HRTEM thus became indispensable.
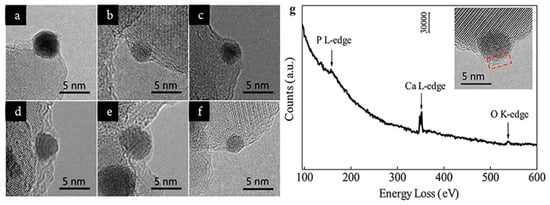
By HRTEM, Tang et al. found that the coverage of HAP on Au nanoparticles depended on the calcination temperature.[8] As shown in Figure 3a–e, the encapsulation layer on the surface of Au nanoparticle appeared when the Au/HAP calcined at 300 °C, then the layer gradually spread as the calcination temperature increased, and finally completely wrapped the Au nanoparticle after being calcined at 600 °C. By subsequent treatment under pure H2 at 500 °C, the encapsulation layer retreated (Figure 3f).
Verifying the composition of the encapsulation layer is critical to concrete the mass transport of the support. For most SMSI or OMSI systems, the encapsulation layer is generally amorphous, which could not be determined by measuring the lattice spacing. The electron energy loss spectrum (EELS) is powerful to analyze the composition and the element valence of materials, thus has long been used in SMSI studies.[16][17][18] For instance, the cover layer on Au nanoparticles in the Au/HAP calcined at 600 oC was detected by EELS and the result (Figure 3g) showed the layer was composed of P, Ca and O, confirming it was derived from the HAP support.[8]
3.3. Electron Transfer
Generally, the electron transfer during OMSI is mainly manifested by the perturbation in the valence state and the change in electronic structure of the supported metal, which can be examined by element-specific spectroscopic techniques that are sensitive to chemical state and atomic structure. Earlier for the research of classical SMSI, X-ray photoelectron spectroscopy (XPS), ultraviolet photoelectron spectroscopy (UPS), and Auger electron spectroscopy (AES) have been employed in characterizing the high-temperature reduction-induced chemical state changes of metal.[19][20][21][16][17][22][23] The aforementioned CO-probed IR spectroscopy can also be used to testify the phenomenon of electron transfer as it is sensitive to the metal electronic structure. On the other hand, the electron transfer that accompanied by the support deformation at interface will inevitably impact the surface local coordination structure of the support. For the oxide support-based catalysts, the SMSI- or OMSI-induced electron perturbation can be verified by electron paramagnetic resonance (EPR), a technique sensitive to species with unpaired electrons such as oxygen vacancies or metal ions in paramagnetic valence states.[24][25][26][27] The metal atom-centered local coordination structure that strongly depends on the interaction between metal and the support can be examined by X-ray absorption spectroscopy (XAS), which is also necessary in SMSI and OMSI studies.[22][28][29]
4. Application and Influence of OMSI
The SMSI effect is able to alter the catalyst properties of the adsorption capacity, interface structure, and the electronic state, thus it has been extensively applied to enhance the catalyst performance for diverse reactions and recognized to be a highly effective method currently.[14][30][31][32][33][34][35][36][37][38][39][40] Admittedly, realizing the appropriate state of SMSI for different reactions is critical and difficult, which is limited by the precondition for the initiation and maintenance of SMSI. For instance, high-temperature reduction or a reductive atmosphere is prerequisite for classical SMSI, therefore the application of classical SMSI in non-reducing conditions is constrained. On this premise, discovery of the OMSI effect will enrich the potential application of the metal–support interaction under specific conditions to a large extent. On the other hand, OMSI truly broadens the territory of metal–support interaction, inspiring more explorations on similar phenomena that occurred in catalysts with multiple metals and supports and evoked by various conditions. This is of great value in in-depth cognition of metal–support interaction and catalytic mechanism.
4.1. Enhancing Catalyst Performance by Tuning the OMSI
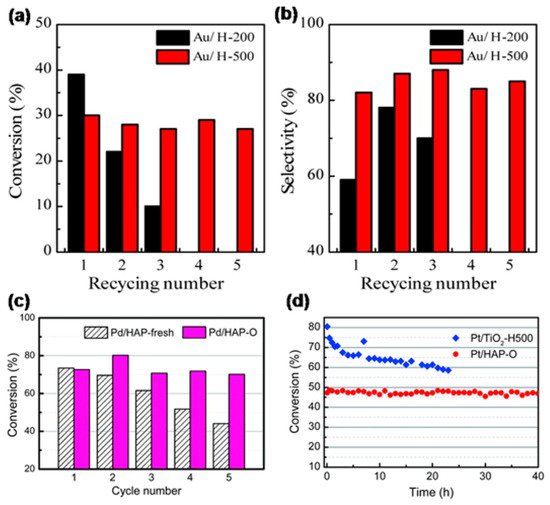
When OMSI was evoked and the encapsulation layer formed, metal nanoparticles were restrained on the support, thus they will be prevented from aggregation or leaching during reaction. Tang et al. compared the recycle performance of Au/HAP catalysts calcined at 200 and 500 °C for the selective oxidation of benzyl alcohol.[8] As shown in Figure 4a,b, both conversion and selectivity of the catalyst calcined at 500 °C (Au/H-500) remained unchanged after five cycles of use, and no Au loss was detected. However, the Au/HAP that calcined at 200 oC (Au/H-200) without encapsulation layer formed has seriously deactivated in the first three reaction cycles, and the used catalyst showed sintered Au nanoparticles and decreased loading. Similarly, reusability of Pd/HAP for Suzuki cross-coupling was significantly enhanced by calcining at 500 oC (Figure 4c).[9] Moreover, as shown in Figure 4d, Pt/HAP calcined at 500 oC (Pt/HAP-O) exhibited excellent durability under a simulated auto-emission control reaction condition, that the CO oxidation conversion kept unchanged during a 40 hour test under 400 oC, which is distinctly better than the Pt/TiO2 reduced at 500 oC (Pt/TiO2-H500, where classical SMSI formed) that deactivated severely in the first 5 hours.[9] These evidently demonstrated the formation of OMSI can effectively inhibit the leaching and aggregation of metal species during reactions in liquid-phase or under elevated temperature with oxidative atmosphere, which is of great importance for practical application.
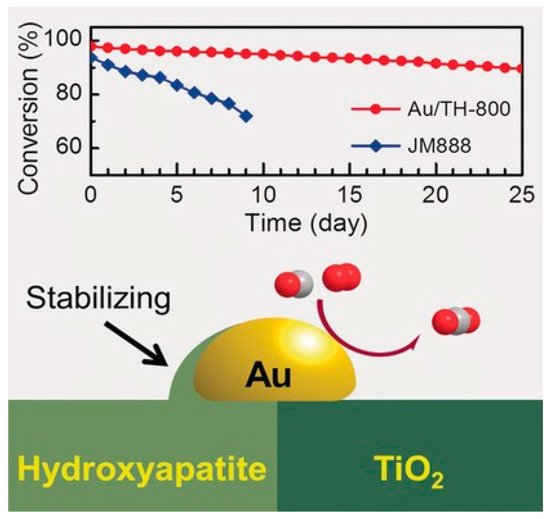
Catalyst activity mostly depends on the interface sites, which would be impacted by encapsulation when SMSI was evoked. Meanwhile, limited by the support type, catalysts that are able to form SMSI may not be particularly active for certain reactions, although the stability can be enhanced. Controlled SMSI state therefore is needed to obtain catalysts with both improved stability and activity. Commendably, Tang et al. developed an ultrastable Au nanocatalyst with high activity by tuning the OMSI between Au and the composite support TiO2-HAP.[10] As shown in Figure 5, the Au nanoparticle was located at the interfacial regions between the TiO2 and HAP.

4.2. Discoveries Inspired by or Based on OMSI
References
- S. J. Tauster; S. C. Fung; R. L. Garten; Strong metal-support interactions. Group 8 noble metals supported on titanium dioxide. Journal of the American Chemical Society 1978, 100, 170-175, 10.1021/ja00469a029.
- S Tauster; S. C. Fung; Strong metal-support interactions: Occurrence among the binary oxides of groups IIA?VB. Journal of Catalysis 1978, 55, 29-35, 10.1016/0021-9517(78)90182-3.
- François Pesty; Hans-Peter Steinrück; Theodore E. Madey; Thermal stability of Pt films on TiO2(110): evidence for encapsulation. Surface Science 1995, 339, 83-95, 10.1016/0039-6028(95)00605-2.
- Y. Gao; Y. Liang; S.A. Chambers; Thermal stability and the role of oxygen vacancy defects in strong metal support interaction — Pt on Nb-doped TiO2(100). Surface Science 1996, 365, 638-648, 10.1016/0039-6028(96)00763-7.
- Lei Zhang; Rajendra Persaud; Theodore E. Madey; Ultrathin metal films on a metal oxide surface: Growth of Au onTiO2(110). Physical Review B 1997, 56, 10549-10557, 10.1103/physrevb.56.10549.
- D. W. Goodman; ?Catalytically active Au on Titania:? yet another example of a strong metal support interaction (SMSI)?. Catalysis Letters 2005, 99, 1-4, 10.1007/s10562-004-0768-2.
- Xiaoyan Liu; Ming-Han Liu; Yi-Chia Luo; Chung-Yuan Mou; Shawn D. Lin; Hongkui Cheng; Jin-Ming Chen; Jyh-Fu Lee; Tien-Sung Lin; Strong Metal–Support Interactions between Gold Nanoparticles and ZnO Nanorods in CO Oxidation. Journal of the American Chemical Society 2012, 134, 10251-10258, 10.1021/ja3033235.
- Hailian Tang; Jiake Wei; Fei Liu; Botao Qiao; Xiaoli Pan; Lin Li; Jingyue (Jimmy) Liu; Junhu Wang; Tao Zhang; Strong Metal–Support Interactions between Gold Nanoparticles and Nonoxides. Journal of the American Chemical Society 2015, 138, 56-59, 10.1021/jacs.5b11306.
- Hailian Tang; Yang Su; Yalin Guo; Leilei Zhang; Tianbo Li; Ketao Zang; Fei Liu; Lin Li; Jun Luo; Botao Qiao; et al.Junhu Wang Oxidative strong metal–support interactions (OMSI) of supported platinum-group metal catalysts. Chemical Science 2018, 9, 6679-6684, 10.1039/c8sc01392f.
- Hailian Tang; Fei Liu; Jiake Wei; Botao Qiao; Kunfeng Zhao; Yang Su; Changzi Jin; Lin Li; Jingyue (Jimmy) Liu; Junhu Wang; et al.Tao Zhang Ultrastable Hydroxyapatite/Titanium‐Dioxide‐Supported Gold Nanocatalyst with Strong Metal–Support Interaction for Carbon Monoxide Oxidation. Angewandte Chemie International Edition 2016, 55, 10606-10611, 10.1002/anie.201601823.
- Hailian Tang; Yang Su; Bingsen Zhang; Adam F. Lee; Mark A. Isaacs; Karen Wilson; Tang Hailian; Wilson Karen; Jiahui Huang; Masatake Haruta; et al.Botao QiaoXin LiuChangzi JinDangsheng SuJunhu WangTao Zhang Classical strong metal–support interactions between gold nanoparticles and titanium dioxide. Science Advances 2017, 3, e1700231-e1700231, 10.1126/sciadv.1700231.
- Liang Wang; Jian Zhang; Yihan Zhu; Shaodan Xu; Chengtao Wang; Chaoqun Bian; Xiangju Meng; Feng-Shou Xiao; Strong Metal–Support Interactions Achieved by Hydroxide-to-Oxide Support Transformation for Preparation of Sinter-Resistant Gold Nanoparticle Catalysts. ACS Catalysis 2017, 7, 7461-7465, 10.1021/acscatal.7b01947.
- Gary L. Haller; Daniel E. Resasco; Metal–Support Interaction: Group VIII Metals and Reducible Oxides. Advances in Catalysis 1989, 36, 173-235, 10.1016/s0360-0564(08)60018-8.
- Xiaorui Du; Yike Huang; Xiaoli Pan; Bing Han; Yang Su; Qike Jiang; Mingrun Li; Hailian Tang; Gao Li; Botao Qiao; et al. Size-dependent strong metal-support interaction in TiO2 supported Au nanocatalysts. Nature Communications 2020, 11, 5811, 10.1038/s41467-020-19484-4.
- A Singh; Electron microscopy study of the interactions of rhodium with titania. Journal of Catalysis 1985, 94, 422-435, 10.1016/0021-9517(85)90207-6.
- Yip-Wah Chung; W. B. Weissbard; Surface spectroscopy studies of the SrTiO3(100) surface and the platinum-SrTiO3(100) interface. Physical Review B 1979, 20, 3456-3461, 10.1103/physrevb.20.3456.
- S Takatani; Strong metal-support interaction in Ni/Ti02: Auger and vibrational spectroscopy evidence for the segregation of TiOx (x $ap;-1) on Ni and its effects on CO chemisorption. Journal of Catalysis 1984, 90, 75-83, 10.1016/0021-9517(84)90086-1.
- Felipe Polo-Garzon; Thomas F. Blum; Zhenghong Bao; Kristen Wang; Victor Fung; Zhennan Huang; Elizabeth E. Bickel; De-En Jiang; Miaofang Chi; Zili Wu; et al. In Situ Strong Metal–Support Interaction (SMSI) Affects Catalytic Alcohol Conversion. ACS Catalysis 2021, 11, 1938-1945, 10.1021/acscatal.0c05324.
- Yip-Wah Chung; Guoxing Xiong; Chia-Chieh Kao; Mechanism of strong metal-support interaction in Ni/TiO2. Journal of Catalysis 1984, 85, 237-243, 10.1016/0021-9517(84)90126-x.
- Hassan Sadeghi; SMSI in Rh/TiO2 model catalysts: Evidence for oxide migration*1. Journal of Catalysis 1984, 87, 279-282, 10.1016/0021-9517(84)90188-x.
- S. J. Tauster; Strong metal-support interactions. Accounts of Chemical Research 1987, 20, 389-394, 10.1021/ar00143a001.
- Bruce C. Beard; Philip N. Ross; Platinum-titanium alloy formation from high-temperature reduction of a titania-impregnated platinum catalyst: implications for strong metal-support interaction. The Journal of Physical Chemistry 1986, 90, 6811-6817, 10.1021/j100284a020.
- S. Roberts; R.J. Gorte; A study of the migration and stability of titania on a model Rh catalyst*1. Journal of Catalysis 1990, 124, 553-556, 10.1016/0021-9517(90)90202-u.
- J. Sanz; J. M. Rojo; P. Malet; G. Munuera; M. T. Blasco; J. C. Conesa; J. Soria; Influence of the hydrogen uptake by the support on metal-support interactions in catalysts. Comparison of the rhodium/titanium dioxide and rhodium/strontium titanate (SrTiO3) systems. The Journal of Physical Chemistry 1985, 89, 5427-5433, 10.1021/j100271a023.
- Catherine Louis; Christine Lepetit; Michel Che; EPR characterization of oxide supported transition metal ions: Relevance to catalysis. Molecular Engineering 1994, 4, 3-38, 10.1007/bf01004048.
- Krystyna Dyrek; Michel Che; EPR as a Tool To Investigate the Transition Metal Chemistry on Oxide Surfaces. Chemical Reviews 1997, 97, 305-332, 10.1021/cr950259d.
- Ning Liu; Ming Xu; Yusen Yang; Shaomin Zhang; Jian Zhang; Wenlong Wang; Lirong Zheng; Song Hong; Min Wei; Auδ−–Ov–Ti3+ Interfacial Site: Catalytic Active Center toward Low-Temperature Water Gas Shift Reaction. ACS Catalysis 2019, 9, 2707-2717, 10.1021/acscatal.8b04913.
- D.R. Short; A.N. Mansour; J.W. Cook Jr.; D.E. Sayers; J.R. Katzer; X-Ray absorption edge and extended x-ray absorption fine structure studies of Pt/TiO2 catalysts. Journal of Catalysis 1983, 82, 299-312, 10.1016/0021-9517(83)90196-3.
- Chengwu Qiu; Yaroslav Odarchenko; Qingwei Meng; Peixi Cong; Martin A. W. Schoen; Armin Kleibert; Thomas Forrest; Andrew M. Beale; Direct observation of the evolving metal–support interaction of individual cobalt nanoparticles at the titania and silica interface. Chemical Science 2020, 11, 13060-13070, 10.1039/d0sc03113e.
- Yaru Zhang; Xiaoli Yang; Xiaofeng Yang; Hongmin Duan; Haifeng Qi; Yang Su; Binglian Liang; Huabing Tao; Bin Liu; De Chen; et al.Xiong SuYanqiang HuangTao Zhang Tuning reactivity of Fischer–Tropsch synthesis by regulating TiOx overlayer over Ru/TiO2 nanocatalysts. Nature Communications 2020, 11, 3185, 10.1038/s41467-020-17044-4.
- Siwei Li; Yao Xu; Yifu Chen; Weizhen Li; Lili Lin; Mengzhu Li; Yuchen Deng; Xiaoping Wang; Binghui Ge; Ce Yang; et al.Siyu YaoJinglin XieYongwang LiXi LiuDing Ma Tuning the Selectivity of Catalytic Carbon Dioxide Hydrogenation over Iridium/Cerium Oxide Catalysts with a Strong Metal-Support Interaction. Angewandte Chemie International Edition 2017, 56, 10761-10765, 10.1002/anie.201705002.
- Jian Li; Yaping Lin; Xiulian Pan; Dengyun Miao; Ding Ding; Yi Cui; Jinhu Dong; Xinhe Bao; Enhanced CO2 Methanation Activity of Ni/Anatase Catalyst by Tuning Strong Metal–Support Interactions. ACS Catalysis 2019, 9, 6342-6348, 10.1021/acscatal.9b00401.
- Carlos Hernández Mejía; Tom W. Van Deelen; Krijn P. De Jong; Activity enhancement of cobalt catalysts by tuning metal-support interactions. Nature Communications 2018, 9, 4459, 10.1038/s41467-018-06903-w.
- Avelino Corma; Pedro Serna; Patricia Concepción; José Juan Calvino; Transforming Nonselective into Chemoselective Metal Catalysts for the Hydrogenation of Substituted Nitroaromatics. Journal of the American Chemical Society 2008, 130, 8748-8753, 10.1021/ja800959g.
- Min-Sung Kim; Sang-Ho Chung; Chun-Jae Yoo; Myung Suk Lee; Il-Hyoung Cho; Dae-Won Lee; Kwan-Young Lee; Catalytic reduction of nitrate in water over Pd–Cu/TiO2 catalyst: Effect of the strong metal-support interaction (SMSI) on the catalytic activity. Applied Catalysis B: Environmental 2013, 142-143, 354-361, 10.1016/j.apcatb.2013.05.033.
- Kazumasa Murata; Daichi Kosuge; Junya Ohyama; Yuji Mahara; Yuta Yamamoto; Shigeo Arai; Atsushi Satsuma; Exploiting Metal–Support Interactions to Tune the Redox Properties of Supported Pd Catalysts for Methane Combustion. ACS Catalysis 2019, 10, 1381-1387, 10.1021/acscatal.9b04524.
- Yaru Zhang; Xiong Su; Lin Li; Haifeng Qi; Chongya Yang; Wei Liu; Xiaoli Pan; Xiaoyan Liu; Xiaofeng Yang; Yanqiang Huang; et al.Tao Zhang Ru/TiO2 Catalysts with Size-Dependent Metal/Support Interaction for Tunable Reactivity in Fischer–Tropsch Synthesis. ACS Catalysis 2020, 10, 12967-12975, 10.1021/acscatal.0c02780.
- Yaru Zhang; Zhen Zhang; Xiaofeng Yang; Ruifeng Wang; Hongmin Duan; Zheng Shen; Lin Li; Yang Su; Runze Yang; Yongping Zhang; et al.Xiong SuYanqiang HuangTao Zhang Tuning selectivity of CO2 hydrogenation by modulating the strong metal–support interaction over Ir/TiO2 catalysts. Green Chemistry 2020, 22, 6855-6861, 10.1039/d0gc02302g.
- Hai Wang; Liang Wang; Dong Lin; Xiang Feng; Yiming Niu; Bingsen Zhang; Feng-Shou Xiao; Strong metal–support interactions on gold nanoparticle catalysts achieved through Le Chatelier’s principle. Nature Catalysis 2021, 4, 418-424, 10.1038/s41929-021-00611-3.
- Bingyu Lin; Biyun Fang; Yuyuan Wu; Chunyan Li; Jun Ni; Xiuyun Wang; Jianxin Lin; Chak-Tong Au; Lilong Jiang; Enhanced Ammonia Synthesis Activity of Ceria-Supported Ruthenium Catalysts Induced by CO Activation. ACS Catalysis 2021, 11, 1331-1339, 10.1021/acscatal.0c05074.
- Hai Wang; Liang Wang; Dong Lin; Xiang Feng; Yiming Niu; Bingsen Zhang; Feng-Shou Xiao; Strong metal–support interactions on gold nanoparticle catalysts achieved through Le Chatelier’s principle. Nature Catalysis 2021, 4, 418-424, 10.1038/s41929-021-00611-3.
- Bingyu Lin; Biyun Fang; Yuyuan Wu; Chunyan Li; Jun Ni; Xiuyun Wang; Jianxin Lin; Chak-Tong Au; Lilong Jiang; Enhanced Ammonia Synthesis Activity of Ceria-Supported Ruthenium Catalysts Induced by CO Activation. ACS Catalysis 2021, 11, 1331-1339, 10.1021/acscatal.0c05074.
