Ultraviolet (UV) sensors offer significant advantages in human health protection and environmental pollution monitoring. Amongst various materials for UV sensors, the zinc oxide (ZnO) nanostructure is considered as one of the most promising candidates due to its incredible electrical, optical, biomedical, energetic and preparing properties. Compared to other fabricating techniques, hydrothermal synthesis has been proven to show special advantages such as economic cost, low-temperature process and excellent and high-yield production.
- zinc oxide nanostructures
- hydrothermal
- localized heat growth
- seed patterned growth
- growth critical parameters
- UV sensors
1. Introduction
Ultraviolet (UV) light provides a special benefit on the well-being of humans by killing microorganisms. However, higher exposure has been reported to cause side effects such as skin cancer, cataracts or immune system suppression. Therefore, sensors that possess the ability of efficiently detecting UV signals have attracted huge attention [1][2][3][1,2,3]. These UV sensors were divided into two groups, including vacuum UV sensors and solid-state UV sensors [4][5][6][4,5,6]. Vacuum UV sensors are based on photomultiplier tubes and their derived devices, whereas solid-state UV sensors are based on semiconductor materials [4]. Compared to solid-state UV sensors, vacuum UV sensors have some disadvantages such as large size, high power consumption, low quantum efficiency, high pressure, low-temperature working conditions and high cost [7]. Therefore, solid-state UV sensors are the new focus for UV technology [4][8][9][10][11][4,8,9,10,11].
In the last few decades, semiconducting metal oxide nanoscale materials were the most likely candidates for electronic, optical, biomedical and thermal applications. They were widely used in UV lasers, sensors, field-effect transistors, field emission devices, energy harvesters, light-emitting sources, phonic devices and nanogenerators [3][4][12][13][14][15][16][17][18][19][20][21][22][3,4,12,13,14,15,16,17,18,19,20,21,22]. These metal oxide materials include zinc oxide (ZnO), nickel oxide (NiO), titanium oxide (TiO 2), copper oxide (CuO), tin oxide (SnO 2), iron oxide (Fe 2O 3), indium oxide (In 2O 3), tungsten trioxide (WO 3) and vanadium oxide (V 2O 3) [1][4][14][17][19][23][24][25][26][27][28][1,4,14,17,19,23,24,25,26,27,28]. Among all of these materials, ZnO has gained considerable interest due to its fascinating unique properties, as mentioned in Table 1 . Its properties include a direct large bandgap (3.37 eV), huge excitation binding energy (60 meV), excellent electron mobility (1 to 200 cm 2/V. s) and huge piezoelectric coefficient (d 33~12 pm/V) [13][16][17][23][29][30][31][13,16,17,23,29,30,31]. Excellent biocompatibility, biodegradability and chemical stability, as well as amazing electrical and optical properties are also some great characteristics of ZnO [12][13][15][17][20][25][32][33][12,13,15,17,20,25,32,33]. Meanwhile, various morphologies of ZnO nanomaterials have been investigated, such as nanoparticles, nanowires, nanoneedles and nanotubes, which accordingly expand their applications in various fields [12][13][14][17][19][34][12,13,14,17,19,34].
| Type of Property | Property |
|---|---|
| Preparation | Easy to grow [12]. Low- and high-temperature operation capability. Architecture and property controllability. Facility of integration on either rigid or flexible devices [20]. |
| Optical | Large bandgap [13]. Good transparency to visible light. Luminescent material [20]. Good transmissibility and reflexibility [35][36]. |
| Electrical | Semiconductor material. Good electron mobility [37]. Good chemical stability [16]. Huge piezoelectric coefficient [11][38]. |
| Biomedical | Excellent biocompatibility [13][20]. Excellent biodegradability [25]. Non-toxicity [33][37]. |
| Energy | Huge excitation binding energy [16][23]. Photocatalytic material [37][39]. |
ZnO is available in three crystalline structures, including wurtzite, zinc blende and rock salt. Wurtzite structure is a two-lattice parameter-based hexagonal unit cell with a = 0.3296 nm and c = 0.52065 nm [13][18][40][13,18,40]. ZnO wurtzite is stable under ambient conditions, but it is transformed into rock salt at relatively high pressure (approximately 10 GPa) [18][41][18,41]. The zinc blende can only be obtained at its stable phase on cubic substrates [20][41][20,41].
Recently, comprehensive work was reported in synthesizing ZnO nanostructures for UV sensors [4][40][42][4,40,76]. Their majority was focusing on various synthesis methods. However, each method possesses various approaches and it was short of a detail interpretation about the mechanism of each method. As economic cost for the synthesis is under increasing challenge, a comprehensive review on each method is of great importance for the future study. To the best of authors’ knowledge, no previous review has precisely examined all of hydrothermal approaches and summarized the effect of critical parameters on the ZnO synthesis. Therefore, the current review aims on the latest approaches of ZnO hydrothermal growths and the effect of different parameters on its morphology. This paper provides an overview of the recent developments in ZnO nanostructure synthesis for UV sensors, particularly the hydrothermal synthesis. Different hydrothermal approaches are compared, and the critical parameters are discussed in detail. Lastly, the hydrothermal ZnO-based UV sensors for UV light are discussed as well. This review may provide a better comprehension of the current research status for hydrothermal ZnO-based applications.
2. ZnO Nanostructures’ Hydrothermal Growth
ZnO is a fascinating material with various morphologies. The chemical and physical characteristics vary as a function of morphology, size, shape and crystalline structures. Previous works have demonstrated that this material can be modelled on the desired shape and size [12][14][17][18][43][44][12,14,17,18,42,77].
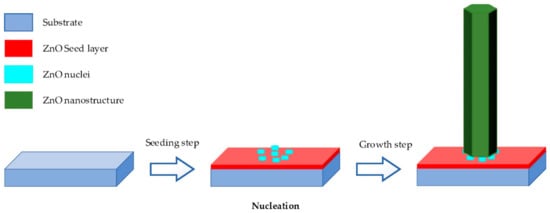
Hydrothermal method is carried out in an aqueous solution by an autoclave system [2][33][34][37][41][2,33,34,37,41]. It contains two typical steps as illustrated in Figure 13 .
-
Seeding: The subtract is seeded with a layer of ZnO nanoparticles. The seeded nanoparticles play a role in promoting nucleation for nanostructure growth by decreasing the thermodynamic barrier. The less the nucleation of ZnO is, the bigger ZnO growth and the better the crystallinity of ZnO [45].
-
Growing: The seeded subtract is kept in the precursor at a certain temperature for a fixed period to ensure stable growing regimes. The precursor is a mixture of aqueous solutions containing alkaline reagent (such as NaOH, KOH and hexamethylenetetramine (HMTA)) and zinc ion salt (such as Zn (NO3)2 and ZnCl2) [39][40][46][47]. In addition to the precursor, a guiding agent (such as polyethyleneimine (PEI)) is inserted to decrease the lateral growth and maximize the length of nanostructures.
Deng et al. employed the hydrothermal process to grow ZnO nanorods on flexible Kapton substrate at low temperature with an equimolar solution of HMTA and zinc nitrate hexahydrate as a precursor [19]. They obtained nanorods in the form of regular hexagonal prisms with a length of 60 nm and a diameter of 100 nm. Vijayakumar et al. presented ZnO nanotubes synthesized by hydrothermal method in autoclave for 4 h at 90 °C for CO gas sensing [48][83]. Hu et al. hydrothermally synthesized ZnO nanowires on polyethylene terephthalate (PET) fabrics at 95 °C for 4 h [34]. The as-grown nanowires were treated hydrophobically by polydimethylsiloxane (PDMS) for lotus effect. Via hydrothermal growth, Wu et al. demonstrated the synthesis of graphene quantum dot doped ZnO superstructures for weak UV intensity sensor application at 90 °C for 2 h [49][80].
In order to control the morphology, orientation, aspect ratio and surface density of the ZnO nanostructure, parameters involved in the process must be optimized. Examples of parameters affecting nanostructure growth morphology are the pH of the solution, reagents, seed layers, temperature, guiding agents, growth time and mechanical agitations [18][50][18,50].
3. Influence of Fabrication Parameters on ZnO Hydrothermal Growth
With cautious adding ammonium hydroxide NH 4OH in the precursor mixture, Boubenia et al. discovered a possibility of enhancing the nucleation sites, which led to the control of nanowires’ electrical properties and expanded the applications for flexible and electromechanical devices [16]. Based on their work, they explained the density-controlled synthesis growth mechanism of ZnO nanowires as follows: The amount of NH 4OH had a straight effect over the concentration of Zn (II) complexes, which would significantly impact the Zn solubility in the solution. Thus, the supersaturation of precursor solution was controlled as well as the quantity of nuclei over the target substrate.
In hydrothermal growth process, growth time is another important critical parameter of ZnO nanostructures [51][52][53][54][47][48,65,87,97,99]. As shown in Table 23 , researchers fabricated ZnO nanostructures with different growth times to set up the relationship between yield of growth and their morphologies.
| ZnO Morphology |
Starting Materials | Synthesis Temperature (°C) |
Growth Time | Diameter of ZnO Nanostructures | Length of ZnO Nanostructures | References |
|---|---|---|---|---|---|---|
| Nanowires | 25 mM of zinc nitrate hexahydrate, 25 mM HMTA and 5–7 mM PEI. | 95 | 1 h | 200–400 nm | 10–12 µm | [55] |
| Nanowires | 25 mM of zinc nitrate hexahydrate, 25 mM HMTA, 5–7 mM PEI and deionized (DI) water. | 95 | 1 h | >20 µm | - | [56] |
| Nanowires | 25 mM of zinc nitrate hexahydrate, 25 mM HMTA and 6 mM PEI. | - | - | - | 9.9 µm | [24] |
| Nanowires | 25 mM of zinc nitrate hexahydrate, 25 mM HMTA and 5–7 mM PEI. | 90 | 2.5 h | 100–150 nm | 1–3 µm | [57] |
| Nanowires | 25 mM of zinc nitrate hexahydrate, 25 mM HMTA and 5–7 mM PEI. | 95 | 1 h | 15 µm | 200–400 nm | [58] |
| Hemispherical bumps | Mixture of equimolar zinc nitrate hexahydrate and HMTA. | 90 | 5 h | 400 nm | 2.2 µm | [59] |
| Nanorods | Mixture of equimolar zinc nitrate hexahydrate and HMTA. | 90 | 3 h | 100 nm | 800 nm | [60] |
| Nanorods | 50 mL of solution containing 0.1 M zinc nitrate hexahydrate, 0.1 M HMTA and DI water. | 90 | 2 h | 70 nm | 15 µm | [26] |
| Nanorods | Mixture of equimolar zinc nitrate hexahydrate and HMTA | 90 | 4 h | 1.2 | - | [2] |
| Flower-like structure | Zinc acetate dehydrate and NaOH. | 120 | 15 min | 0.6 µm | 5.2 µm | [32] |
| Nanowires | ZnCl2. NaCO3 and DI water. | 140 | 6 h | 50 nm | 1 µm | [52] |
| Vertical aligned nanorods |
Zn(CH3COO)2·2H2O, HMTA, absolute ethanol and distilled water. |
400 | - | 50 nm | 500 nm | [13] |
| Nanorods | 10 mL Zn(Ac)2.2H2O in 0.1 M methanol, 20 mL NaOH in 0.5 M methanol, DI water (K2SnO3,3H2O, 95%), 0.75 g of urea. | 150 | 24 h | 2.8 nm | 26 nm | [43] |
| 20 mM Zn(NO3)2 and 20 mM HMTA |
90 °C for 100 min, dried for 12 h at 60 °C and annealed 1 h at 500°C. | - | 290–330 nm | 3.2–3.4 µm |
The type of synthesized ZnO nanostructures is also affected by the temperature. On the Zn foil substrate, Lu et al. synthesized well-aligned ZnO nanorods of 30 nm in diameter and 200 nm in length at 22 °C and ultralong ZnO nanowires arrays with honeycomb-like structures of 60 to 200 nm in diameter and 10 to 30 mm in length at elevated temperature under similar conditions [61][108].
In hydrothermal method, ZnO nanostructures were treated with thermal annealing after either the seed deposition or the growth in order to alter their properties [1][60][62][63][1,106,109,110]. For instance, Filip et al. reported a significant difference in crystalline structure on a seed layer between annealed and non-annealed substrates [35]. Lupan et al. demonstrated that post-treatment thermal annealing led to improvement in the crystallinity and the performance of ZnO nanomaterials [64][82]. Sandeep Sanjeev and Dhananjaya Kekuda also showed that annealing temperature affected the structural and optical properties of the ZnO thin film [36]. Wahid et al. reported that the optimum annealing temperature was 150 °C, where they obtained high-resistant ZnO nanorods with a length and diameter of 4000 nm and 379 nm, respectively [65][86]. They also discovered that the ZnO growth rate depended on the annealing temperature, as vertical nanorods were observed below 150 °C and ZnO homocentric bundles on the vertical nanorods above 150 °C. Through careful analysis of the seed layer, Wahid et al. explained the mechanism behind this observation as follows. At annealing temperatures above 150 °C, more energy was present in the seed layer, which enhanced the kinetic energy of the seed layer molecule. In consequence, the molecular motion increased and this caused the seed layer to stretch more and reduce the surface tension. As a result, the seed nanoparticles agglomerated, which then brought the nanoparticles together during the annealing process. As the seed nanoparticles were agglomerating, the active nucleation sites of ZnO seed were disorientated, resulting in multifarious ZnO nanorod growth orientation, which promoted bundling of the ZnO nanorods [65][66][86,111]. Meanwhile, Wei et al. reported that this agglomeration phenomenon happened because annealing produced the dried organic compound (diethanolamine) [67][112].