Phytomedicines exist either in isolated or purified form or as a mixture of different secondary metabolites, and are used to prevent and cure different diseases. Phytomedicines may also have vitamins and minerals which are believed to synergize preventive and therapeutic effects, and additionally be useful in treatment of drug-resistant cancers.
- phytomedicines
- phytochemicals
- stem cells
- signaling pathway
- epithelial–mesenchymal transition
- preclinical
- clinical research
1. Introduction
Cancer is one of the deadly diseases affecting the population worldwide, despite advancements in numerous therapeutic interventions. One of the major problems in cancer treatment is drug resistance, and cancer stem cells (CSCs) have been found as one of the important mediators to impart resistance [1][2][1,2]. CSCs are the drug-resistant cells that possess a unique ability for self-renewal, which makes them immortal [3]. There are numerous pluripotency-associated transcription factors, such as Oct4, Sox2, and Nanog, which play an essential role in maintaining the stemness of these CSCs [4]. Due to the stemness, CSCs lead to tumor heterogeneity and aggressiveness, which eventually results in metastasis [5]. CSCs also impart the dormancy of the tumors that causes treatment resistance and increases the chance of relapse [6]. CSCs are responsible for the initiation and progression of cancer as well as recurrence after treatment. Thus, CSCs have generated interest in understanding cancer treatment and prognosis in recent years [7][8][7,8]. CSCs account for EMT, which makes the cells more motile and invasive. Aberration or dysregulation of various molecular and cellular signaling pathways as well as altered metabolism of CSCs and dysregulated EMT further exacerbate the tumor heterogeneity. Altogether, the evidence points out that CSCs play a crucial role in cancer dissemination, from initiation to progression and relapse [9].
Since cancer is a fatal disease affecting millions of people worldwide, there is a great necessity of different treatment options to overcome the drug resistance or recurrence conditions in cancer patients. For the treatment of cancer, the conventional modalities are radiotherapy and chemotherapy. However, in recent years, accumulating experimental and epidemiologic studies have demonstrated that the plants and plant-derived bioactive agents, popularly known as phytochemicals, showed medicinal value, and appear beneficial as chemo-preventive and chemotherapeutic agents. Medicinal plants and their bioactive compounds have been a very good and easily accessible source for the development of novel therapeutics for various cancer diseases. Many of them have also been found to exert chemo-sensitizing effects and synergize the anticancer effects, and thus may be useful in drug-resistant cancer cells. Collectively, the plant-based formulations available either as a single herb formulation, or a mixture of many plant extracts or the plant-derived compounds, are called phytomedicines, and these have attracted interest to be future candidates in cancer therapy, owing to their selective cytotoxicity against cancer cells as well as fewer or negligible adverse effects [2]. Phytomedicines exist either in isolated or purified form or as a mixture of different secondary metabolites, and are used to prevent and cure different diseases [10]. Phytomedicines may also have vitamins and minerals which are believed to synergize preventive and therapeutic effects, and additionally be useful in treatment of drug-resistant cancers [10]. The plant extracts or plant-derived bioactive constituents have been tested for several years and showed anti-tumor activity by modulating the dysregulated signaling pathways, targeting efflux pump or transporters, and/or inducing apoptotic cell death and cell cycle arrest. Even though several purified bioactive phytocompounds and crude extracts of thousands of medicinal plants have been tested for their therapeutic effects during cancer disease treatment, very few of them have been studied on both in vitro and in vivo platforms, and only a few of them are under clinical trials.
In recent years, the utilization of many plant extracts and plant-derived agents has gained momentum for their activity against CSCs. The available studies are indicative of their anti-CSC properties mediating the modulation of numerous signaling pathways, which participate in the physiological and molecular regulation of CSCs. Many studies have shown that medicinal plants, plant-derived bioactive compounds, or the plant formulations commonly used in traditional Chinese medicines (TCM) reduce the stem-like characteristics of CSCs. They exhibit their activities by interfering with EMT genes, reducing invasiveness, and inhibiting migratory properties of CSCs [11]. In purview of the increasing understanding of the role of CSCs in many cancer types, in the present review, we comprehensively discussed the recent studies showing targeting of the CSCs by phytomedicines. The mechanisms and effects are presented in synoptic tables and schemes. The present review is suggestive of the therapeutic opportunities and prospects of phytomedicines and encouraging further studies for their pharmaceutical development.
2. Phytomedicines Targeting Key Regulators of Anti-Cancer Drug Resistance in CSCs
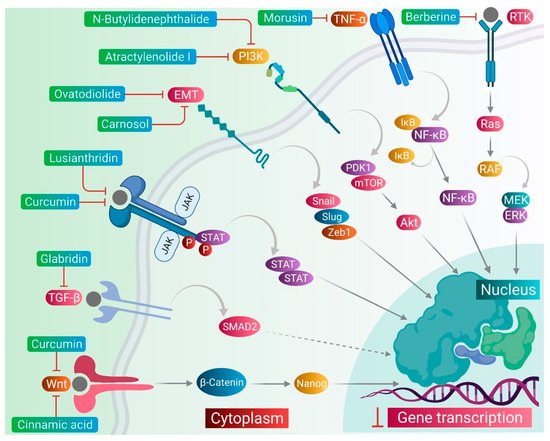
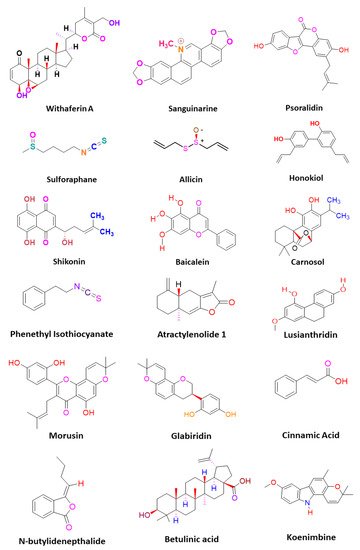
CSCs contribute to the anticancer drug resistance by numerous mechanisms, including EMT regulation, enhanced expression of ABC transporters, overexpression of aldehyde dehydrogenase (ALDH) enzyme, slow cycling of microRNAs, regulation of tumor microenvironment, as well as resistance to DNA damage and cell death [12][15]. Phytomedicines target any one of these key regulating machineries in the resistance of CSCs to the anticancer drugs ( Figure 1 ). The targeting of these mechanisms could be important in ceasing or eliminating CSCs and it may improve the outcome of anticancer drugs during chemotherapy of cancer. Some of these phytomedicines are discussed in this section and their related information and figures are provided in Figure 2 and summarized in Table 1 .
| Plant Source | Extract | Bioactive Compound | Mode of Action | In Vivo Dose | Cells/Model | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alstonia scholaris | Fruit extract | Scholarisine Q(1) and R(2) |
| ------ | Glioma stem cells | [13] | [40] | ||||
| Anisomeles indica | ------ | Ovatodiolide |
| ------ | Glioblastoma stem-like cells | [14] | [26] | ||||
| ------ | CSC population in nasopharyngeal carcinoma | [15] | [23] | |||||||
| 3.6 mg/kg | Oral CSCs and xenograft tumor mice | [16] | [24] | |||||||
| Cruciferous vegetables |
------ | Phenethyl isothiocyanate |
| 20 mg/kg | Colon CSCs and xenograft tumor mice | [17] | [41] | ||||
| 10 µM | Cervical CSCs and Xenograft NOD-SCID tumor mice | [18] | [42] | |||||||
| Atractylodes macrocephala Koidz | Rhizome extract | Atractylenolide I |
| 25 mg/kg and 75 mg/kg | Stemness of colon cancer cells and xenograft tumor mice | [19] | [43] | ||||
| Fructus viticis | ------ | Flavonoids |
| ------ | Lung CSCs | [20] | [44] | ||||
| Pigeon pea | ------ | Cajaninstilbene acid derivatives |
| ------ | Breast cancer stem-like cells | [21] | [45] | ||||
| Berberis libanotica Ehrenb | Root extract | ------ |
| ------ | Prostate CSCs | [22] | [46] | ||||
| Berberis | , | Arcangelisia Hydrastis | ------ | Berberine |
| ------ | Stemness in neuroblastoma cells | [23] | [32] | ||
| Dendrobium | venustum | Stem extract | Lusianthridin |
| ------ | Lung CSCs | [24] | [22] | |||
| Curcuma longa | ------ | Curcumin |
| ------ | Breast CSCs | [25] | [47] | ||||
| ------ | Curcumin |
| ------ | Breast CSCs | [26] | [48] | |||||
| ------ | Curcumin |
| ------ | Papillary thyroid CSCs | [27] | [49] | |||||
| ------ | Curcumin |
| 5 mg/kg | Glioblastoma stem cells and xenograft tumor mice | [28] | [50] | |||||
| Walsura pinnata Hassk | Bark extract | Betulonic acid |
| 18, 36, or 54 μM | Leukemia stem cells and xenotransplanted zebrafish | [29] | [16] | ||||
| Costus speciosus | Rhizome extract | ------ |
| ------ | Stemness of prostate cancer cells | [30] | [51] | ||||
| Viola odorata | Hydro-alcoholic extract of aerial part | ------ |
| ------ | Breast CSCs | [31] | [52] | ||||
| Polygonum cuspidatum | Root extract | 2-Ethoxystypandrone |
| ------ | Hepatocellular CSCs | [32] | [53] | ||||
| Cinnamomum cassia | ------ | Cinnamic acid |
| ------ | Colon CSCs | [33] | [19] | ||||
| Glycyrrhiza glabra | ------ | Glabridin |
| 20 mg/kg | Breast cancer stem-like cells and xenograft tumor mice | [34] | [54] | ||||
| Morus australis | ------ | Morusin |
| ------ | Cervical CSCs | [35] | [55] | ||||
| Lithospermum erythrorhizon | ------ | Shikonin |
| 2 mg/kg | Glioblastoma stem cells and xenograft tumor mice | [36] | [56] | ||||
| Rosmarinus officinalis | ------ | Carnosol |
| ------ | Glioblastoma CSCs | [37] | [57] | ||||
| PienTze Huang | ------ | ------ |
| ------ | Colorectal CSCs | [38] | [58] | ||||
| Allium sativum | ------ | Allicin (diallyl thiosulfinate) |
| ------ | Melanoma stem-like cells | [39] | [25] |
Lusianthridin, a phenanthrene derivative and phenolic compound isolated from the stem of the plant Dendrobium venustum, was shown to downregulate the Src-STAT3-c-Myc signaling pathway and suppress CD133, ABCG2, and ALDH1A1 stemness markers, which induced the apoptosis in lung CSCs [24][22]. The root extract of Polygonum cuspidatum consisting of 2-ethoxystypandrone, i.e., a novel analogue of juglone, was shown to exhibit inhibition of the STAT3 signaling pathway in hepatocellular carcinoma cells (HCC cells). It ceased the growth and proliferation of HCC cells in a dose-dependent manner and induced the programmed cell death of CSCs in HCC [32][53].
Cinnamic acid, a monocarboxylic acid isolated from the bark of cinnamon ( Cinnamomum zeylanicum ), has been shown to decrease the stemness of colon CSCs [33][19]. Shikonin, a naphthoquinone derivative abundantly found in the roots of a Chinese medicinal herb, Lithospermum erythrorhizon, modulated the JNK/c-Jun pathway and augmented its cytotoxicity in glioblastoma stem cells (GSCs). The findings observed in the in vitro studies were confirmed in a GSCs-xenografted mice model [36][56]. Morusin, a flavonoid present in the roots of Morus australis, was found to attenuate NF-κB activity in cervical CSCs and induced apoptotic cell death in these CSCs by curbing their migration and growth. Furthermore, this compound exerted cytotoxicity of the cervical CSCs [35][55]. Glabridin, an isoflavane obtained from the roots of Glycyrrhiza glabra, modulated epigenetic regulation of miR-148a/SMAD2 signaling and inhibited stem cell-like properties of human breast cancer cells. Additionally, Glabridin (20 mg/kg) improved the survival of breast cancer mouse xenografts [34][54]. Similarly, Cajaninstilbene acid derivatives of pigeon pea modulated the cytotoxicity (pathway not deduced) in breast cancer stem-like cells [21][45].
Similar to the plant-derived bioactive compounds, plant extracts have been tested in many studies. The crude extracts prepared from whole plant or certain plant parts showed potent anticancer effects in many cancer types by targeting drug-resistant CSCs in their cell populations. For example, the bark extract of Walsura pinnata Hassk and the rhizome extract of Costus speciosus induced the apoptotic cell death in the leukemic and prostate CSCs, respectively [29][30][16,51]. In the next sections, we comprehensively discuss targeting of several stemness markers, EMT genes, and cellular signaling pathways, which could be an important therapeutic approach for the elimination of CSCs from the drug-resistant cancer cell populations, as well as to keep a check on the cell growth and proliferation, therefore ceasing the tumor growth.
3. Phytomedicines Targeting Wnt/β-Catenin, Notch, and Hedgehog Signaling in CSCs
As we discussed earlier, the Wnt, Notch, and Hh signaling pathways are responsible for the stem-like characteristics of cancer cells and account for their self-renewal [11]. Therefore, we primarily focused on reviewing the phytomedicines which particularly target these pathways. Therapeutic targeting of these pathways by phytomedicines could pave the design and development of natural therapeutics. Phytomedicines targeting these pathways are schematically elucidated in Figure 3 .
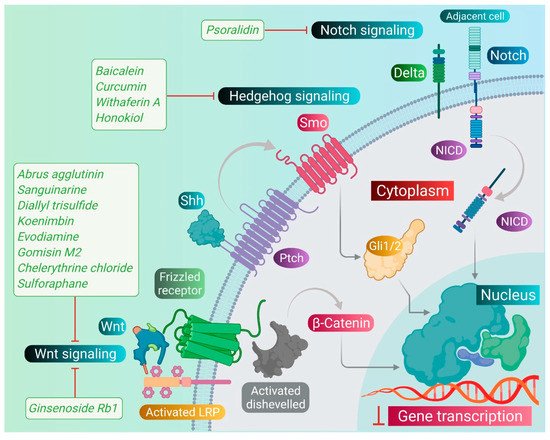
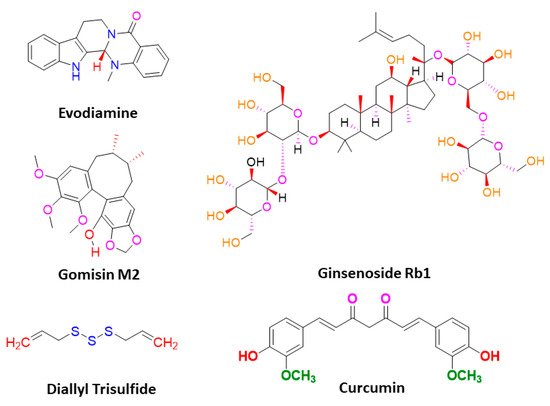
Sanguinarine, a benzophenanthridine alkaloid obtained from Chelidonium majus L. Plant (traditional Chinese medicine celandine), downregulated the Wnt/β-catenin signaling pathway and inhibited the proliferation and invasion of lung CSCs, thereby inducing the apoptosis in lung CSCs [40][67]. Gomisin M2, a lignan belonging to the class of hydrolysable tannins, also forms an active component of a Chinese medicine Baizuan, and inhibited the proliferation of MDA-MB-231 and HCC1806 cells. Additionally, it suppressed the self-renewal potential of breast CSCs by downregulating the Wnt/β-catenin signaling pathway. Consequently, it blocked the formation of mammospheres in breast CSCs and induced apoptosis in breast CSCs by altering mitochondrial membrane potential [41][68]. Furthermore, evodiamine, a natural quinolone alkaloid isolated from Evodia rutaecarpa, inhibited the proliferation of bulk cultured colon cancer cells and arrested cell cycle at G2/M phase, thereby inducing apoptosis in these cells. Further, this compound repressed the expression of several genes regulating the key signaling pathways such as Notch and Wnt of colon CSCs, and eliminated these cells [42][69]. Moreover, evodiamine also inhibited the proliferation and induced apoptosis in gastric CSCs. This compound also decreased the expression of pluripotent stem cell markers such as Bmi-1, KLF4, Sox2, and Oct4, and EMT markers such as Slug, Zeb1, Twist, and vimentin. The observations demonstrate that evodiamine exerts an inhibitory effect on the Wnt/β-catenin signaling pathway and EMT, thus suppressing the proliferation and stem cell-like properties of gastric CSCs [43][70].
Like the Wnt signaling pathway, the Notch pathway also maintains the stemness of CSCs. A traditional Chinese medicine (TCM), Qingyihuaji formula (QYHJ), composed of different Chinese herbs, including Herba Scutellariae Barbatae , Herba Hedyotdis , Herba seu Radix Gynostemmatis Pentaphylli , Rhizoma Arisaematis Erubescentis , and Fructus Amomi Rotundus, was found to target the Notch signaling pathway. This formulation reduced the CD133 expression on pancreatic CSCs. Additionally, it downregulated the expression of the Notch-4 gene, but in combination with gemcitabine, it significantly suppressed expression of Notch-1, Notch-2, and Notch-3 genes, too. Furthermore, QYHJ, at the dose of 36 g/kg, inhibited tumor growth in SW1990 cells xenografted tumor mice models, suggesting that QYHJ possesses the potential to increase the survival time of patients by reducing pancreatic CSCs [44][71]. Similarly, another TCM formulation, Xiaotan Sanjie (XTSJ), composed of many herbs, inhibited the cell viability of gastric CSCs in a dose-dependent manner, attributed to the downregulation of Notch-1 expression, i.e., regulating the proliferation of gastric CSCs. Further, XTSJ at different doses (1.46, 2.92, and 5.84 g/mL) reduced the tumor growth in a dose-dependent manner in gastric CSC-transplanted mice models [45][72]. Another very popular TCM preparation, Pien Tze Huang (PZH), decreased the percentages of side population cells in SW480 cells. Additionally, it reduced the viability of side population cells in a dose-dependent manner and induced apoptosis in these side population cells, as evidenced by fragmented nucleus and condensed chromatin. Subsequently, PZH downregulated the Notch1 gene expression in colon CSCs, demonstrating its action as a potent agent targeting CSC [46][73]. Next, Psoralidin, a prenylated coumestans derivative isolated from the seeds of Psoralea corylifolia, inhibited Notch-1 signaling in breast CSCs that promoted the inhibition of EMT markers. This resulted in decreased invasion and migration of ALDH+ breast CSCs. Further, it inhibited the growth and induced programmed cell death in breast CSCs [47][74].
The Hh signaling pathway also plays an important role in maintaining the stemness of CSCs. Additionally, numerous studies demonstrated that deregulation of the Hh pathway plays an important role in tumorigenesis as well as drug resistance in a multitude of cancers by driving cancer cell proliferation, malignancy, metastasis, and the expansion of CSCs. The targeting of this signaling pathway by phytomedicines may inhibit the proliferation and growth of CSCs. Withaferin A, a lactone isolated from the leaf extract of Withania somnifera, inhibited the transcriptional activity of the GLI1-DNA complex formed during the Hh signaling pathway in different CSCs. This compound exhibited potent cytotoxicity against PANC1, DU145, and MCF7 cancer cells [48][75]. One of the popular phytocompounds curcumin, isolated from rhizomes of Turmeric ( Curcuma longa ), was shown to inhibit the Sonic Hh pathway and reduce the expression of breast CSC markers (ALDH1, CD44, OCT4, and CD133). This causes cessation of the cell proliferation and induces the apoptotic cell death in breast CSCs [49][76]. Furthermore, another constituent, sulforaphane, isolated from cruciferous vegetables including broccoli, has been shown to block the Sonic Hh pathway (Smo, Gli-1, 2) and reduce the markers of EMT (Zeb-1), pluripotency (Oct4, Nanog), angiogenic (VEGF, PDGFRα), and metastasis in pancreatic CSCs. This leads to the induction of apoptosis in pancreatic CSCs, and thus significantly reduced the tumor growth in pancreatic CSC-transplanted NSG mice [50][18].
