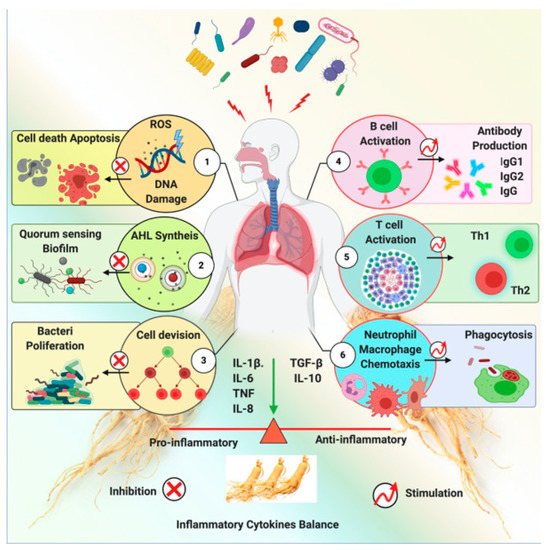
Ginseng has been reported to inhibit bacterial pathways, thereby killing bacteria indirectly. It has also been shown to protect the host from bacterial invasion.
- ginseng
- respiratory tract infection
- immuno-modulatory effects
- cytokines
- antiviral activity
- antibacterial activity
1. Introduction
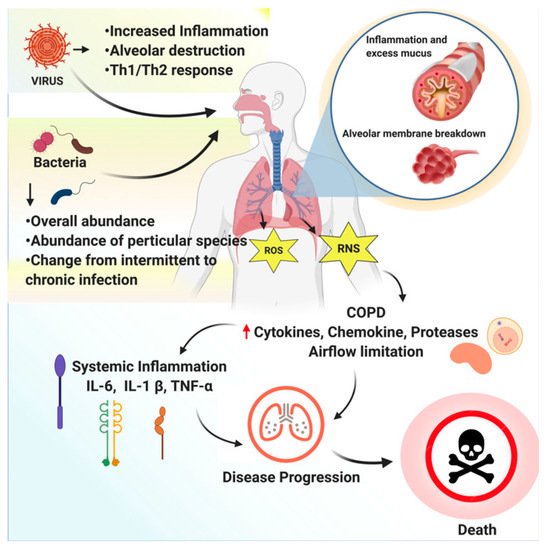
According to MedlinePlus, lung disease is considered any problem in the lungs that prevents them from working correctly. The standard classifications of lung diseases are restrictive, obstructive, or vascular. WHO estimates that the third most comprehensive reason for death worldwide by 2030 may be chronic obstructive pulmonary disease (COPD). The majority of infections are caused by cosmopolitan agents, while geographical or tropical infections are rare.
In clinical medicine, respiratory tract infections (RTIs) are considered prevalent and pose vital problems. Antibiotics are commonly prescribed to treat and manage respiratory infections, even though published literature indicates that they rarely benefit patients. Nasal pharyngitis, acute bronchitis, and non-specific upper respiratory tract infections are caused by respiratory viruses [1]. Several different types of viruses may infect the respiratory tract; these include the adenovirus, rhinovirus, parainfluenza virus, coronavirus, enterovirus, respiratory syncytial virus, and influenza virus.
RTIs are divided into upper respiratory tract infections (throat and sinuses) and lower respiratory tract infections (airways and lungs). To date, the medical practitioners’ primary focus has been on the antagonists that inhibit the recruitment and activation of inflammatory cells. However, none of the currently available anti-inflammatory medications provide satisfactory relief to COPD patients and may end up producing side effects; therefore, safe, effective medications for inhibiting inflammatory response are needed to treat COPD [2]. An overview of respiratory tract infections caused by bacteria or viruses is depicted inFigure 1.
For thousands of years, herbal drugs have been used to cure numerous illnesses and to improve overall well-being. Among the commonly used herbal medicines,Panax ginsengC. A. Meyer is a recognized herb cultivated mainly in Korea, China, and the U.S.A. The principal ingredients of ginseng are amino acids, proteins, flavonoids, volatile oils, and polysaccharides [3][4]. Various forms of ginseng are available, including fresh, dried, boiled, and red ginseng, as well as extracts.
In the past 50 years, numerous clinical and preclinical research studies have been conducted on ginseng [5][6]. However, few studies have exploredP. ginsengagainst COPD and other associated disorders, such as chronic bronchitis, but these have shown encouraging results [7][8][9][10]. The key active component of ginseng was first established by Shibata et al. The active constituents’ composition and quality depend on various factors, such as the method of cultivation, harvesting season, preservation method, age, and part of the plant used [11].
Human immune cells were treated with various ginseng extracts by Lau et al. The observed anti-inflammatory role of ginseng was attributed to the combined effects of these ginsenosides targeting different immunological activity levels, thereby contributing to ginseng’s various actions in humans [12]. Studies conducted on animals have shown that ginseng provokes a robust immune response that protects against bacterial and viral infections [13][14][15]. The role of ginseng and its main active constituents in reducing the risk and continuation of flu and colds has been reported in several studies, including clinical trials [16].
Herein, we reviewed the available literature on ginseng’s active components and their role against respiratory pathogens. The present review summarized ginseng’s possible modes of action, clinical evidence, and consequences as a therapeutic agent against respiratory infections. Interventional clinical trials are needed to evaluate ginseng’s properties, including immunomodulatory, anti-inflammatory, antimicrobial, and antiviral activities.
2. Ginseng Structural Features
The primary structural moiety of ginseng saponins is a hydrophobic, four trans-ring rigid steroidal skeleton [17]. Ginsenosides are saponins that are derivatives of triterpene dammarane. [18]. Most of the studies have focused on the role of ginsenosides, rather than ginseng extract, for treating diseases [4][19][20][21][22][23][24].
3. Anti-Bacterial Activity of Ginseng
Microbial infections have various causes, and the resulting diseases require different antibiotics as treatment. However, the improper use of antibiotics is the cause of resistance and toxic side effects, as well as the emergence of multidrug-resistant bacteria, which is now a global health emergency [25]. In the absence of newer antibiotics, natural products are being promoted to address this issue. Ginseng has been reported to inhibit bacterial pathways, thereby killing bacteria indirectly.
Ginseng exhibits a shielding effect against the inflammation induced by a pathogen. Ginseng exerts this effect via several mechanisms, including anti-quorum sensing, inhibition of pathogen-induced hemagglutination, DNA mutagenesis, and immune-modulatory functions. An impression of ginseng’s antibacterial activity is shown in Figure 6. Ginseng and its derived components’ anti-bacterial effects are represented inTable 2.
| Ginseng Extracts and Compounds | Microbe | Study Type | Observations | Conclusions | Reference | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Withaferin A (WA), a withanolide purified from | Withania somnifera | H. pylori | In vitro study | WA inhibits | H. pylori | -induced IL-8 production in gastric epithelial cells. | WA does not influence | H. pylori | -induced ROS production or any associated signaling. | [27] | |||||||||||||||
| Withania somnifera | (Indian ginseng), Both aqueous as well as alcoholic extracts of the plant (root as well as leaves) | Pathogenic bacteria | In vitro study | Inhibitory activity against a spectrum of bacteria. | Increased survival rate as well as decreased bacterial load. | [28] | |||||||||||||||||||
| Withania somnifera | (Indian ginseng) extracts | Salmonella typhimurium | and | Escherichia coli | . | In vitro study | Methanol and hexane extracts of both leaves and roots were found to have potent antibacterial activity. | A synergistic increase in the antibacterial effect of Tibrim was noticed when MIC of Tibrim was supplemented with these extracts. | [29] | ||||||||||||||||
| Extracts of | Withania somnifera | (Indian ginseng) | Staphylococcus aureus | , | Escherichia coli | , | Pseudomonas aeruginosa | and | Bacillus subtilis | In vitro study | Polar solvents had higher antibacterial property in comparison with the nonpolar solvents; higher MIC values were obtained for both gram-positive bacteria | S. aureus | , | B. subtilis | and gram-negative bacteria, | E. coli | and | P. aeruginosa | , with polar extract. | Antimicrobial activity of crude extract of | W. somnifera | was shown to validate the use of traditional medicinal herbal medicine and results of this study tend to give credence to the common use of | W. somnifera | plant. | [30] |
| P. ginseng | polysaccharides | H. pylori | hemagglutination and enzyme-linked glycosorbent assays | Acidic carbohydrates may play an important role in the inhibitory activity on | H. pylori | adhesion to host cells. | Bacterial binding was inhibited more effectively by | P. ginseng | polysaccharides | [31] | |||||||||||||||
| Fermented ginseng extracts | H. pylori | Formation of clear zones, measurement of urease activity and cell adhesion activity in vitro. | Anti- | H. pylori | activity, including anti-bacterial, anti-adhesion, and urease inhibition effects. | Fermented ginseng extract containing | L.plantarum | MG 208 could prove to be useful as a functional diet for the protection of the gastric environment against | H. pylori. | [32] | |||||||||||||||
| Red ginseng extracts (RGE) | H. pylori | Analysis of cell viability (trypan blue dye exclusion assay, DNA fragmentation assay (comet assay) Measurement of cytokine level, cell signaling (in vitro) | RGE decreased H. pylori-stimulated IL-8 gene expression, which resulted from the transcriptional regression of NF-κB. | RGE showed significant gastroprotective effects against | H. pylori | -associated gastric mucosal cell damage, suggesting that red ginseng could be used as a medicinal phytonutrient against | H. pylori | infection. | [33] | ||||||||||||||||
| White ginseng extract (WGE) | H. pylori | Disc diffusion assay | The zone of inhibition due to WGE increased significantly with increasing dosage. WGE exhibited an inhibitory effect on cell growth at 2.0 mg/mL for all tumor cell lines. | The potential of WGE to be used as a health-promoting substance. | [34] | ||||||||||||||||||||
| Ginseng aqueous extract | Pseudomonas aeruginosa | P. aeruginosa | biofilms were further investigated in vitro and in vivo. | Oral administration of ginseng extracts in mice promoted phagocytosis of | P. aeruginosa | PAO1 by airway phagocytes but did not affect phagocytosis of a PAO1-film mutant. | Ginseng treatment may help to eradicate the biofilm-associated chronic infections caused by | P. aeruginosa. | [35] | ||||||||||||||||
| Saline extract of ginseng | Pseudomonas aeruginosa | Cytokine modulating effect in a mouse model of | P. aeruginosa | lung infection. | Th1-like immune response in the mice with | P. aeruginosa | lung infection after 7 days of ginseng treatment. | Th1 response might benefit the host with | P. aeruginosa | lung infection and ginseng treatment might be a promising alternative measure for the treatment of chronic | P. aeruginosa | lung infection in CF patients. | [36] | ||||||||||||
| Polysaccharide (PS) isolated from | Panax ginseng | Staphylococcus aureus | In vitro assays for the activity measurement of PS, NO production test with Greiss reagent, in vivo anti-septicemic activity was assessed by using C57BL/6J mice. | Polysaccharide showed anti-septic effects, Ginsan enhanced pro-inflammatory abilities (NO, pro-inflammatory cytokine production, phagocytic activity of macrophages). Ginsan modulated TLR pathway. | PS from | Panax ginseng | possess a potent anti-septicemic activity by stimulating macrophage and potential as an immunomodulator against sepsis caused by | Staphylococcus aureus. | [37] | ||||||||||||||||
| Polysaccharide (PS) isolated from | Panax ginseng | Staphylococcus aureus | In vitro study | Proinflammatory cytokines, such as TNF-alpha, IL-1beta, IL-6, IFN-gamma, IL-12, and IL-18, were markedly down-regulated in ginsan-treated mice compared with those of control-infected mice. | Antiseptic activity of ginsan can be attributed to enhanced bacterial clearance, and reduced proinflammatory cytokines via the TLR signaling pathway. | [38] | |||||||||||||||||||
| Korean red ginseng | Staphylococcus aureus | Fluorescent marker calcein from negatively charged PC/PG (1: 1, | w | / | w | ) liposomes | Ginsenosides may exert antibacterial activity by disrupting the cell membrane | Synergistic or additive effects between the ginsenosides and antibiotics tested | [39] | ||||||||||||||||
| Crude saponins extracted from the | Panax quinquefolius | Fusobacterium nucleatum, Clostridium perfringens, and Porphyromonas gingivalis | Determination of MIC, cell integrity | HTS, HTS-3, and HTS-4 were effective at inhibiting the growth | of F. nucleatum | , | C. perfringens | , and | P. gingivalis | . | Less polar ginsenoside-enriched fraction from heat transformation can be used as an antibacterial agent to control halitosis. | [40] | |||||||||||||
| Acidic polysaccharide from | P. ginseng | , PG-F2 | P. gingivalis | Determination of MIC | Anti-adhesive activity and anti-hemagglutination. | PG-F2 may exert a selective antiadhesive effect against pathogenic bacteria, while having no effects on beneficial and commensal bacteria. | [41] | ||||||||||||||||||
| A mixture of roasted coffee and red ginseng | Pseudomonas aeruginosa | and | S. Typhimurium | Classical paper disc method | DPPH scavenging activity decreased when red ginseng extract composed of more than 70% of the total extract. | Antibacterial activity shown. | [42] |
Abbreviations: WA: Withaferin A; MIC: minimum inhibitory concentration; CF: cystic fibrosis; TLR: toll-like receptor; DPPH: 2,2 diphenyl-1-picryl-hydrazyl-hydrate; TNF-alpha: Tumor Necrosis Factor Alpha; ROS: reactive oxygen species; NF-Kb: Nuclear Factor kappa; MBC: minimum bactericidal concentration; KRG: Korean red ginseng; RGE: red ginseng extract; NO: nitric oxide; PC: Phosphatidylcholine; PG; Phosphatidyl glycerol; HTS: heat-transformed saponins; HTS-3 & HTS 4: Ginsenoside enriched fractions.Pseudomonas is commonly found in soil, water, and the environment. When people come in contact with this contaminated water or soil, they become infected [26][43]. While multiple types ofPseudomonasexist,Pseudomonas aeruginosacauses most of the infections in humans. This type causes infection in the lungs (pneumonia), but it has evolved to circumvent the effects of the antibiotics used to treat it [16][26][44].
P. ginsengaqueous extract was administered by subcutaneous injection at a dose of 25 mg/kg of body weight per day, along with saline as a control. The ginseng-treated infected group showed a higher IgG2a level and lower IgG1 level than the control group. The variations in IgG1 and IgG2a subclasses imply a possible shift from a Th-2- to a Th-1 response. The findings of this study suggested that the effect ofP. ginsengcould be related to the activation of a Th-1 type of cellular immunity and down-regulation of humoral immunity [45].P. ginsengmight also be considered an add-on therapy to treat cystic fibrosis, as it can reduce bacterial infections and biofilm formation.
Another study was conducted to investigate the antimicrobial activity of the aqueous extract ofPanax quinquefoliusfrom North American ginseng (NAGE) root againstPseudomonas aeruginosa. MIC (minimum inhibitory concentrations) of reference andPseudomonas aeruginosa’s clinical isolates were measured by a standard agar dilution method. The extract eradicated six-day-old mature biofilms (5%w/v), while luorescence microscopy displayed a reduction of live cells and biofilm complexes compared with non-treated biofilms [46].
Ginseng is a complex mixture of several components, some of which enhance bacterial growth, while others repress it. Previous studies via animal models showed that ginseng treatment offered protection from chronic lung infection caused byP. aeruginosa. However, an aqueous extract of ginseng in concentrations of 0.5–2.0% did not inhibitP. aeruginosa, but it did significantly limit the formation ofP. aeruginosa’s biofilm. This was suggested as a possible mechanism noted in a previous study by which ginseng helped the bacterial clearance from animal lungs in vivo.
These functions deregulate the humoral immune response and lessen the formation of immune complexes [47][48]. Ginseng could play a vital role in combating microbial infections, particularly againstP. aeruginosapneumonia. PMNs are a common cause of cystic fibrosis, the leading cause of morbidity and mortality [49][50]. Thus, ginseng shows good therapeutic activity
Most of theS. pneumoniaeproduce diseases; a few of the serotypes cause most of the pneumococcal infections. The human respiratory tract has commensal Pneumococcus, which is the cause of local infections, as well as many invasive diseases, such as meningitis and sepsis, due to its virulence factors. Additionally, pre-treated mice showed lower morbidity and bacterial numbers. It thereby strengthens cell continuance against pneumococcal infection [51].
Korean red ginseng extract’s protective effect against pneumococcal infection and sepsis have been investigated. Colonization, survival rate and body weight were calculated. Mice treated with 100 mg/kg of KRG had significantly higher survival rates and body weights than those of the non-treated controls. A dosage of 100 mg/kg of KRG protected the host cells from fatal pneumococcal sepsis by inhibiting inflammation and intensifying bacterial clearance, augmenting cell survival against the pneumococcal infection [52].
4. Ginseng Clinical Trials
In this section, summaries of human clinical trials from various databases, such as lens.org and clinicaltrial.org, are presented. No formal inventory has been created showing ginseng in the context of respiratory diseases. Ginseng products are generally used as complementary and alternative medicine in respiratory infections. More research is needed to explore the uses of ginseng in the context of respiratory diseases.
Results have shown that ginseng relieves the symptoms and prevents respiratory infections. COLD-fX has been isolated from the roots of American ginseng. It is effective and safe against respiratory pathogens, as well as in reducing the viral load of patients who are prone to seasonal influenza. The immunomodulatory constituents of COLD-fX act through toll receptors and influence a rise in cell numbers and functions in innate and adaptive immune systems [53].
A randomized, double-blinded trial investigated the effectiveness of COLD-fX in acute respiratory illness (ARI). After the dosing of COLD-fX in mice in vitro, COLD-fX (CVT-E002) was reported to cause a significant increase in lymphocyte proliferation and cytokine production (IL-1, IL-6, TNF-α, and nitric oxide) from peritoneal macrophages. The extract’s ability to stimulate IL-2 and IFN-γ release could be attributed to its efficacy against respiratory infections. It was found that COLD-fX was safe and reduced the severity and incidence of upper respiratory tract infections [54].
studied ginseng’s efficacy in preventing common colds in healthy adults. A systematic review of randomized controlled trials or controlled clinical trials comparing Asian ginseng (Panax ginseng) and North American (Panax quinquefolius) These five trials investigated only North American ginseng and the trials differed in their methodological quality. However, in comparison with the placebo groups, ginseng medications reduced common cold symptoms by 25%.
Predy et al. studied the efficacy of North American ginseng containing poly-furanosyl-saccharides in preventing upper respiratory tract infections. Participants were given two capsules of North American ginseng extract or placebo daily for four months. A moderate dose of North American ginseng for four months lessened the number of colds per person. The results also showed that participants who had two or more prior cold symptoms had less-severe symptoms than those with no prior symptoms [55].
Mono-preparations of ginseng behave as a placebo, as reported in several clinical trials. Isolated cases reported more serious adverse events, but it is difficult to provide evidence of casualty. Ginseng as an add-on therapy has shown severe adverse events and even casualties; however, after reviewing all the cases, it is difficult to conclude thatP. ginsengcould cause the problems. Combination therapy does appear to be more closely associated with adverse events [10][56].
A pilot study of a randomized, controlled trial was conducted to evaluate the efficacy of GINST and GS-3K8 modified ginseng extracts in acute respiratory illness. ginseng(G115) dose of 100 mg twice daily for 12 weeks improved the pulmonary function test of respiratory endurance in 92 patients with COPD [57]. In two groups of patients [(n = 37) (n = 38)], the first group was given 875 mg amoxicillin and 125 mg clavulanic acid, while the second group was given an anti-bacterial treatment with 100 mg standardized ginseng extract G115 twice daily for nine days. Those patients who have complicated bacterial clearance may receive benefit from ginseng [9].
They studied the role of ginseng extract in improving the quality of life and providing symptomatic relief. The trial also suggested that ginseng treatment was safe and had remedial value, as it provided symptomatic relief in patients with COPD [58]. As a remedial treatment in respiratory infections, ginseng shows potential for the development of new herbal medicines. More effective clinical trials are still needed to prove the potency and effectiveness of ginseng against respiratory infections.
References
- WHO. WHO Technical Report Series 954 Evaluation of Certain Veterinary Drug Residues in Food Seventieth Report of the Joint FAO/WHO Expert Committee on Food Additives Food and Agriculture Organization of the United Nations World Health Organization; WHO: Geneva, Switzerland, 2009; ISBN 9789241209540.
- Cazzola, M.; Page, C.P.; Calzetta, L.; Matera, M.G. Emerging anti-inflammatory strategies for COPD. Eur. Respir. J. 2012, 40, 724–741.
- Hyun, S.H.; Kim, S.W.; Seo, H.W.; Youn, S.H.; Kyung, J.S.; Lee, Y.Y.; In, G.; Park, C.K.; Han, C.K. Physiological and pharmacological features of the non-saponin components in Korean Red Ginseng. J. Ginseng Res. 2020, 44, 527–537.
- Ishihara, Y.; Takemoto, T.; Ishida, A.; Yamazaki, T. Protective actions of 17 β -Estradiol and progesterone on oxidative neuronal injury induced by organometallic compounds. Oxid. Med. Cell. Longev. 2015, 2015, 1–16.
- Jia, L.; Zhao, Y.; Liang, X.-J. Current Evaluation of the Millennium Phytomedicine- Ginseng (II): Collected Chemical Entities, Modern Pharmacology, and Clinical Applications Emanated from Traditional Chinese Medicine. Curr. Med. Chem. 2009, 16, 2924–2942.
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693.
- An, X.; Zhang, A.L.; Yang, A.W.; Lin, L.; Wu, D.; Guo, X.; Shergis, J.L.; Thien, F.C.K.; Worsnop, C.J.; Xue, C.C. Oral ginseng formulae for stable chronic obstructive pulmonary disease: A systematic review. Respir. Med. 2011, 105, 165–176.
- Gross, D.; Krieger, D.; Efrat, R.; Dayan, M. Ginseng extract G115® for the treatment of chronic respiratory diseases. Scweiz. Z. Ganzheits. Med. 1995, 1, 29–33.
- Scaglione, F.; Weiser, K.; Alessandria, M. Effects of the standardised ginseng extract G115® in patients with chronic bronchitis: A nonblinded, randomised, comparative pilot study. Clin. Drug Investig. 2001, 21, 41–45.
- Coon, J.T.; Ernst, E. Panax ginseng. Drug Saf. 2002, 25, 323–344.
- Leung, K.W.; Wong, A.S. Pharmacology of ginsenosides: A literature review. Chin. Med. 2010, 5, 1–7.
- Ginseng: Nature’s Anti-Inflammatory?—ScienceDaily. Available online: (accessed on 1 December 2020).
- Lee, J.S.; Lee, Y.N.; Lee, Y.-T.; Hwang, H.S.; Kim, K.-H.H.; Ko, E.-J.J.; Kim, M.-C.C.; Kang, S.-M.M. Ginseng Protects Against Respiratory Syncytial Virus by Modulating Multiple Immune Cells and Inhibiting Viral Replication. Nutrients 2015, 7, 1021–1036.
- Silvestrini, P.; Beccaria, C.; Pereyra, E.A.L.; Renna, M.S.; Ortega, H.H.; Calvinho, L.F.; Dallard, B.E.; Baravalle, C. Intramammary inoculation of Panax ginseng plays an immunoprotective role in Staphylococcus aureus infection in a murine model. Res. Vet. Sci. 2017, 115, 211–220.
- Zhuo, X.; Sun, H.; Wang, S.; Guo, X.; Ding, H.; Yang, Y.; Shan, Y.; Du, A. Ginseng stem-and-leaf saponin (GSLS)-Enhanced protective immune responses induced by Toxoplasma gondii heat shocked protein 70 (HSP70) against toxoplasmosis in mice. J. Parasitol. 2017, 103, 111–117.
- Iqbal, H.; Rhee, D. kwon Ginseng alleviates microbial infections of the respiratory tract: A review. J. Ginseng Res. 2020, 44, 194–204.
- Lü, J.M.; Jiang, J.; Jamaluddin, M.S.; Liang, Z.; Yao, Q.; Chen, C. Ginsenoside Rb1 blocks ritonavir-induced oxidative stress and eNOS downregulation through activation of estrogen receptor-beta and upregulation of SOD in human endothelial cells. Int. J. Mol. Sci. 2019, 20, 294.
- Shin, K.C.; Oh, D.K. Classification of glycosidases that hydrolyze the specific positions and types of sugar moieties in ginsenosides. Crit. Rev. Biotechnol. 2016, 36, 1036–1049.
- Lee, C.H.; Kim, J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res. 2014, 38, 161–166.
- CHENG, Y.; SHEN, L.; ZHANG, J. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol. Sin. 2005, 26, 143–149.
- Zhou, W.; Chai, H.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. Molecular mechanisms and clinical applications of ginseng root for cardiovascular disease. Med. Sci. Monit. 2004, 10, RA187–RA192.
- Lim, K.H.; Lim, D.J.; Kim, J.H. Ginsenoside-Re ameliorates ischemia and reperfusion injury in the heart: A hemodynamics approach. J. Ginseng Res. 2013, 37, 283–292.
- Buettner, C.; Yeh, G.Y.; Phillips, R.S.; Mittleman, M.A.; Kaptchuk, T.J. Systematic review of the of ginseng on cardiovascular risk factors. Ann. Pharmacother. 2006, 40, 83–95.
- Gillis, C.N. Panax ginseng pharmacology: A nitric oxide link? Biochem. Pharmacol. 1997, 54, 1–8.
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. V Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51.
- Nguyen, N.H.; Nguyen, C.T. Pharmacological effects of ginseng on infectious diseases. Inflammopharmacology 2019, 27, 871–883.
- Kim, G.; Kim, T.H.; Kang, M.J.; Choi, J.A.; Pack, D.Y.; Lee, I.R.; Kim, M.G.; Han, S.S.; Kim, B.Y.; Oh, S.M.; et al. Inhibitory effect of withaferin A on Helicobacter pylori-induced IL-8 production and NF-κB activation in gastric epithelial cells. Mol. Med. Rep. 2016, 13, 967–972.
- Owais, M.; Sharad, K.S.; Shehbaz, A.; Saleemuddin, M. Antibacterial efficacy of Withania somnifera (ashwagandha) an indigenous medicinal plant against experimental murine salmonellosis. Phytomedicine 2005, 12, 229–235.
- Arora, S.; Dhillon, S.; Rani, G.; Nagpal, A. The in vitro antibacterial/synergistic activities of Withania somnifera extracts. Fitoterapia 2004, 75, 385–388.
- Sundaram, S.; Dwivedi, P.; Purwar, S. In vitro Evaluation of Antibacterial Activities of Crude Extracts of Withania somnifera (Ashwagandha) to Bacterial Pathogens. Asian J. Biotechnol. 2011, 3, 194–199.
- Lee, J.H.; Eun, K.P.; Uhm, C.S.; Chung, M.S.; Kyung, H.K. Inhibition of Helicobacter pylori adhesion to human gastric adenocarcinoma epithelial cells by acidic polysaccharides from Artemisia capillaris and Panax ginseng. Planta Med. 2004, 70, 615–619.
- Yang, J.W.; Choi, S.Y.; Park, S.J.; Paek, N.S.; Kim, S.S. Anti-Helicobacter Pylori effect of fermented ginseng extracts with Lactobacillus plantarum MG 208. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 53–56.
- Park, S.; Yeo, M.; Jin, J.H.; Lee, K.M.; Jung, J.Y.; Choue, R.; Sung, W.C.; Hahm, K.B. Rescue of Helicobacter pylori—Induced cytotoxicity by red ginseng. Dig. Dis. Sci. 2005, 50, 1218–1227.
- Jee, H.-S.; Chang, K.-H.; Moon, S.-H.; Park, S.-H.; Paik, H.-D. Anti-Helicobacter pylori, Cytotoxic, and Anti-inflammatory Activities of White Ginseng Extract. Food Sci. Biotechnol. 2008, 17, 1106–1109.
- Wu, H.; Lee, B.; Yang, L.; Wang, H.; Givskov, M.; Molin, S.; Høiby, N.; Song, Z. Effects of ginseng on Pseudomonas aeruginosa motility and biofilm formation. FEMS Immunol. Med. Microbiol. 2011, 62, 49–56.
- Song, Z.; Moser, C.; Wu, H.; Faber, V.; Kharazmi, A.; Høiby, N. Cytokine modulating effect of ginseng treatment in a mouse model of Pseudomonas aeruginosa lung infection. J. Cyst. Fibros. 2003, 2, 112–119.
- Lim, D.S.; Bae, K.G.; Jung, I.S.; Kim, C.H.; Yun, Y.S.; Song, J.Y. Anti-septicaemic effect of polysaccharide from Panax ginseng by macrophage activation. J. Infect. 2002, 45, 32–38.
- Ahn, J.-Y.; Choi, I.-S.; Shim, J.-Y.; Yun, E.-K.; Yun, Y.-S.; Jeong, G.; Song, J.-Y. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. Eur. J. Immunol. 2006, 36, 37–45.
- Sung, W.S.; Lee, D.G. The combination effect of Korean red ginseng saponins with kanamycin and cefotaxime against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 2008, 31, 1614–1617.
- Xue, P.; Yao, Y.; Yang, X.S.; Feng, J.; Ren, G.X. Improved antimicrobial effect of ginseng extract by heat transformation. J. Ginseng Res. 2017, 41, 180–187.
- Lee, J.H.; Shim, J.S.; Lee, J.S.; Kim, M.K.; Chung, M.S.; Kim, K.H. Pectin-like acidic polysaccharide from Panax ginseng with selective antiadhesive activity against pathogenic bacteria. Carbohydr. Res. 2006, 341, 1154–1163.
- Choi, Y.H.; Kim, S.E.; Huh, J.; Han, Y.H.; Lee, M.J. Antibacterial and antioxidative activity of roasted coffee and red ginseng mixture extracts. J. Korean Soc. Food Sci. Nutr. 2012, 41, 320–326.
- Pseudomonas aeruginosa Infection | HAI | CDC. Available online: (accessed on 29 January 2021).
- Kim, Y.R.; Yang, C.S. Protective roles of ginseng against bacterial infection. Microb. Cell 2018, 5, 472–481.
- Song, Z.; Kharazmi, A.; Wu, H.; Faber, V.; Moser, C.; Johansen, H.K.; Rygaard, J.; Høiby, N. Effects of ginseng treatment on neutrophil chemiluminescence and immunoglobulin G subclasses in a rat model of chronic Pseudomonas aeruginosa pneumonia. Clin. Diagn. Lab. Immunol. 1998, 5, 882–887.
- Alipour, M.; Omri, A.; Suntres, Z.E. Ginseng aqueous extract attenuates the production of virulence factors, stimulates twitching and adhesion, and eradicates biofilms of Pseudomonas aeruginosa. Can. J. Physiol. Pharmcol. 2011, 89, 419–427.
- Lu, C.C.; Chen, M.Y.; Lee, W.S.; Chang, Y.L. Potential therapeutic agents against COVID-19: What we know so far. J. Chin. Med. Assoc. 2020, 83, 534–536.
- Yoo, D.G.; Kim, M.C.; Park, M.K.; Song, J.M.; Quan, F.S.; Park, K.M.; Cho, Y.K.; Kang, S.M. Protective effect of korean red ginseng extract on the infections by H1N1 and H3N2 influenza viruses in mice. J. Med. Food 2012, 15, 855–862.
- Horsley, A. Book review: Hodson and Geddes’ Cystic Fibrosis. Breathe 2016, 12, 91–92.
- Ramsey, D.M.; Wozniak, D.J. Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Mol. Microbiol. 2005, 56, 309–322.
- Ahmed, A.B.M. Microbial toxinology for safer drug industry. J. Pharm. Care Health Syst. 2016, 03, 4.
- Nguyen, C.T.; Luong, T.T.; Lee, S.Y.; Kim, G.L.; Kwon, H.; Lee, H.G.; Park, C.K.; Rhee, D.K. Panax ginseng aqueous extract prevents pneumococcal sepsis in vivo by potentiating cell survival and diminishing inflammation. Phytomedicine 2015, 22, 1055–1061.
- Wang, M.; Guilbert, L.J.; Ling, L.; Li, J.; Wu, Y.; Xu, S.; Pang, P.; Shan, J.J. Immunomodulating activity of CVT-E002, a proprietary extract from North American ginseng (Panax quinquefolium). J. Pharm. Pharmacol. 2001, 53, 53.
- McElhaney, J.E.; Simor, A.E.; McNeil, S.; Predy, G.N. Efficacy and Safety of CVT-E002, a Proprietary Extract of Panax quinquefolius in the Prevention of Respiratory Infections in Influenza-Vaccinated Community-Dwelling Adults: A Multicenter, Randomized, Double-Blind, and Placebo-Controlled Trial. Influenza Res. Treat. 2011, 2011, 1–8.
- Predy, G.N.; Goel, V.; Lovlin, R.; Donner, A.; Stitt, L.; Basu, T.K. Efficacy of an extract of North American ginseng containing poly-furanosyl-pyranosyl-saccharides for preventing upper respiratory tract infections: A randomized controlled trial. CMAJ 2005, 173, 1043–1048.
- Ernst, E. Panax ginseng: An Overview of the Clinical Evidence. J. Ginseng Res. 2010, 34, 259–263.
- Song, Z.; Kong, K.F.; Wu, H.; Maricic, N.; Ramalingam, B.; Priestap, H.; Schneper, L.; Quirke, J.M.E.; Høiby, N.; Mathee, K. Panax ginseng has anti-infective activity against opportunistic pathogen Pseudomonas aeruginosa by inhibiting quorum sensing, a bacterial communication process critical for establishing infection. Phytomedicine 2010, 17, 1040–1046.
- Xue, C.C.; Shergis, J.L.; Zhang, A.L.; Worsnop, C.; Fong, H.; Story, D.; Da Costa, C.; Thien, F.C.K. Panax ginseng C.A Meyer root extract for moderate Chronic Obstructive Pulmonary Disease (COPD): Study protocol for a randomised controlled trial. Trials 2011, 12, 1–6.
