Beta2-microglobulin (B2M) is a key component of major histocompatibility complex class I molecules, which aid cytotoxic T-lymphocyte (CTL) immune response. B2M also plays an important role in innate defense and does not only function as an adjuvant for CTL response.
- beta2-microglobulin
- antimicrobial peptide
- amyloid fibrils
- cytotoxicity
1. Beta-2 Microglobulin
B2M is characterized as a scaffolding protein that retains the native structure of MHC class I molecules in antigen present cells (APCs) to present peptides to cytotoxic CD8+ T cells. Because MHC class I molecules can affect many physiological functions in mice and human beings, animal models of an engineered B2M deficiency with low MHC class I levels address major challenges in the endogenous cross-presentation of the host [5,6,7][1][2][3]. In addition to being a part of non-membrane-bound proteins associated with MHC class I molecules, B2M can be released in free form in serum [10[4][5],11], a feature of the deposition of amyloid fibrils in the skeletal muscular system.
2. Antimicrobial Activity of B2M
The role of B2M in adaptive immunity has been extensively studied, but its role in innate defense has been neglected. Roch et al. discovered that five earthworm defense proteins exhibited compositional similarity with mouse B2M, reflecting an evolutionary function of B2M in innate defense among invertebrates [13][6]. The antibacterial activity of B2M was first discovered in human amniotic fluid a decade ago [14][7], indicating that potassium ions are critical for the antibacterial activity of B2M. B2M causes the dissipation of bacterial transmembrane potential without damaging the cell membrane and it exhibits a wide range of antibacterial activity against not only antibiotic-susceptible but also antibiotic-resistant bacteria, including L. monocytogenes, E. coli, and S. aureus [14][7]. Due to its antigen presentation role in APCs, B2M-knockout in animal models indicates that immune cells, except for those carrying the T-cell receptor type, can also play an important role against pathogenic infection [15][8]. In addition, D’Souza et al. discovered that a non-class B2M-restricted mechanism affects the early accumulation of lymphocytes and resistance to Mycobactrium tuberculosis (M. tuberculosis) in mice. They also observed that mice had a ability to fight pulmonary infection with tuberculosis when this mechanism was absent [16][9]. The alternative role of B2M in monocyte filtration had not been further investigated until the discovery that B2M acts as a precursor of antibacterial chemokine in human respiratory epithelial cells [20][10]. A 9-kDa fragment derived from B2M, named sB2M-9, was identified in the culture medium of human respiratory epithelial cells (REC) upon IL-1β stimulation and presents in the nasal fluid from healthy individuals [20][10]. The mechanism underlying the shedding of sB2M-9 from REC remains unknown.
Many functional peptides derived from their precursor sequences are known to be released through a proteolytic mechanism. For example, hCAP18, a human antimicrobial peptide (AMP) precursor, harbors an active domain, namely LL-37 in its C-terminal sequence [21][11]. A recent study found that a carboxyl terminal fragment of beta-subunit hemoglobin exhibits antimicrobial activity in the placenta for protection against bacterial and viral infection [22][12].
In a recent study, Holch et al. demonstrated that B2M exerts pH-dependent antimicrobial activity against B. subtilis, P. aeruginosa, and L. monocytogenes in human broncho-alveolar-lavage fluid [25][13]. This finding indicates that the antimicrobial activity of B2M localizes to its C-terminal domain, a region responsible for the formation of fibrils. In that study, the antimicrobial activity of B2M increased as pH decreased for pH < 5.5, but decreased as pH increased for pH > 6, suggesting that B2M exhibits antimicrobial activity under acidic conditions. Therefore, we can infer that an increased dose of B2M at pH 4.5 mediates the formation of fibrils and may thus contribute to the AMP effect; infection is typically associated with a low pH environment (Figure 1).
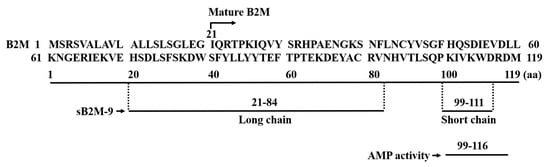
Figure 1. Diagram for the sequences of B2M and B2M-derived peptides with AMP activity. Mature B2M is a polypeptide of 99 amino acid residues (21-119). sB2M-9 [20][10] is composed of long chain (21–84) and short chain (99–111) amino acid sequences as indicated by a dashed line, cross-linking with a disulfide bond (not shown). Sequence 99–116 (theoretical pI = 10.06) with AMP activity [25][13] is shown in comparison with the short chain sequence (99–111, theoretical pI = 9.51) deduced from sB2M-9. One-letter code is used for the amino acid sequence of the peptide.
We recently analyzed a panel of cationic peptides cultured in A549 medium infected with IL-1β by proteomic approach, thioredoxin was especially prominent in addition to some other immune responsible factors, such as cytokines and chemokines (Table 1). Thioredoxin, a small redox protein and a chemokine [30][14], has been discovered to regulate a spectrum of physiological functions, including influencing a disulfide bond-linked dimer of Tapsin:Rep57 within the MHC class I peptide-loading complex [31][15]. In a recent study, the substantially enhanced antimicrobial activity of hBD-1 due to a reduction of disulfide bonds also indicated the key role of thioredoxin in innate defense [28][16]. In summary, considering the evidence for the antimicrobial activity of B2M_99-116 fragment, this study hypothesized that the short chain peptide B2M_99-111 derived from sB2M-9 plays a role in host defense as an AMP. Thus, whether an unrecognized reduction reaction by thioredoxin sheds the short chain fragment from sB2M-9 warrants investigation.
| UniProt Entry | Protein Hit | Cytokine/Chemokine/AMP |
|---|---|---|
| VIME_human | Vimentin | Modulating cytokine [32][17] |
| ALBU_human | Serum albumin | Inducing chemokine synthesis [33][18] |
| IBP1_human | Insulin-like growth factor-binding protein 1 | None |
| CXCL6_human | C-X-C motif chemokine 6 | Chemokine/AMP [34][19] |
| THIO_human | Thioredoxin | Chemokine [30][14] |
| CXCL5_human | C-X-C motif chemokine 5 | Chemokine [35][20] |
| TIMP1_human | Metalloproteinase inhibitor 1 | MMP1 inhibitor: Regulating AMP shedding [36][21] |
| TPM1_human | Tropomyosin alpha-1 chain | None |
| TPM3_human | Tropomyosin alpha-3 chain | None |
| TPM4_human | Tropomyosin alpha-4 chain | None |
| K2C8_human | Keratin, type II cytoskeletal 8 | None |
| IL6_human | Interleukin-6 | Cytokine/Chemokine [37][22] |
| TMEM2_human | Transmembrane protein 2 | None |
| B2MG_human | β-2-microglobulin | Chemokine/AMP [20,25][10][13] |
| LUZP1_human | Leucine zipper protein 1 | None |
| IL8_human | Interleukin-8 | Cytokine/chemokine/AMP [27,38][23][24] |
| FETA_human | α-fetoprotein | A modulator of the pro-inflammatory response [39][25] |
| IBP6_human | Insulin-like growth factor-binding protein 6 | Chemokine [40][26] |
| CH10_human | 10 kDa heat shock protein, mitochondrial | None |
| REL_human | Proto-oncogene c-Rel | Cytokine regulator [41][27] |
| DEST_human | Destrin | None |
| COF2_human | Cofilin-2 | None |
3. pH-Dependent Antimicrobial Activity of B2M
AMPs can have a pH dependent mode of action in a wide range of multicellular organisms [42][28]. In general, they exhibit antimicrobial activity through interacting with the component of the cell membrane to disrupt the membrane’s integrity. After inserting itself into the lipid bilayer of the membrane, AMPs self-aggregate to form pores on the membrane to kill the bacterium [43][29]. A different group of AMPs exhibited optimal activity against microbes at acidic pH conditions; however, some of them were activated at a basic range (Table 2). The different pH values in different tissues were therefore considered to function as a natural barrier against microbial infection.
| Source | AMP | Antimicrobial Activity at Acidic pH | References |
|---|---|---|---|
| Human | B2M | Increase | [25][13] |
| Human | LL-37 | Decrease | [51][30] |
| Human | β-defensin 3 | Decrease | [51][30] |
| Human | β-microseminoprotein | Increase | [52][31] |
| Human | Hepcidin-20, -25 | Increase | [53][32] |
| Human | Lysozyme | Decrease | [54][33] |
| Human | Lactoferrin | Increase | [55][34] |
| Human | Psoriasin (S100A7) | Increase | [56][35] |
| Human | Phagocytin | Increase | [57][36] |
| Human | Dermcidin-1L (DCD-1L) | Increase | [58][37] |
| Human | Hemoglobin β subunit (aa 112–147) |
Increase | [22][12] |
| Human | Calprotectin | Decrease | [59][38] |
| Synthetic | A cationic, amphiphilic random copolymer | Decrease | [60][39] |
| Synthetic | Histidine-rich peptides (histatin) | Increase | [61][40] |
4. Role of Aggregated B2M in Antimicrobial Activity and Cytotoxicity
A study reported the binding of IgG to staphylococcus and to the gram-positive streptococcus groups A, C, and G [62][41]. The role of a potential AMP for B2M was also reported by Kronvall et al.; specifically, they demonstrated that only aggregated B2M with a molecular weight higher than 100,000 Da binds to the surface protein structure in streptococcus groups A, B, and G [63][42]. Apparently, monomeric B2M exhibits no binding effect. Notably, the effect of aggregated B2M on group A1 almost reached maximum binding after only 10 min of incubation. Considering the structural similarity between B2M and the constant (Fc) domains of human IgG and given that IgG binds to staphylococcal protein A, scholars have also investigated the binding effect for B2M on the bacteria. We previously observed that sB2M-9 instead of intact B2M had an SA-binding effect, with no crossing reaction for the IgG Fc domain [20][10]. In addition, we demonstrated that macrophage-activating lipopeptide-2 (MALP-2) but not lipopolysaccharide (LPS) induces sB2M-9 shedding, which is the same effect as that induced by SA instead of Klebsiella pneumonia (a gram-negative strain), implying an affinity for Fc-binding in gram-positive bacteria. Eventually, this effect triggers monocytes migration to the bacterial clumps. AMPs can self-aggregate to facilitate their insertion into bacterial membrane through a pore-forming mechanism [64][43]. Because sB2M-9 shedding is mechanistically similar to that of other AMPs, whether the sB2M-9-mediated effect on SA binding is associated with a monomer, a dimer, an oligomer or highly ordered amyloid fibrils remains unknown and requires further study.
In essence, a variety of potential factors can contribute to the formation of B2M amyloid fibrils, including the concentration of free-form B2M, an acidic pH, the truncation and cleavage by proteolysis, sequence mutation, the presence of metals (especially those with a divalent cation), posttranslational modification (PTM), and lipid interaction [65][44].
Because a beta-sheet rich molecule such as B2M exhibits biological effects that differ depending primarily on the concentration and pH environment, a sB2M-9 concentration of 5 μg/mL is present in the culture medium of human REC, which is close to that in human nasal fluid and other biological tissues [20,74][10][45]. This concentration is also similar with those of other AMPs that exhibit channel forming activity [75][46]. Caution must be exercised when interpreting in vitro finding obtained using doses that are higher than their physiological counterparts and findings obtained under acidic pH, which enables B2M to disrupt microbe membranes. This finding seems to indicate the amyloid-mediated cytotoxicity of B2M fibrils rather than the effect of AMP [11,25][5][13].
5. Conclusions
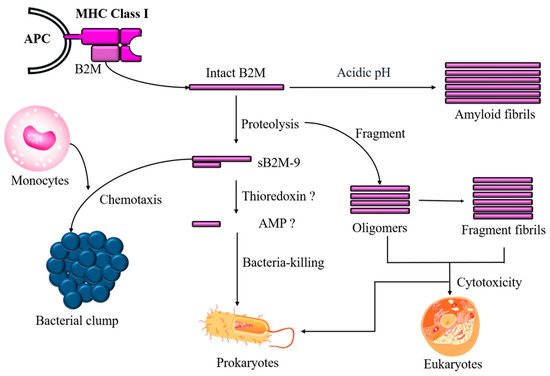
In addition to sB2M-9 shedding, a fragment with a high pI value hidden in B2M that governs antimicrobial activity could be released in response to a microbial challenge. Moreover, intact or fragmented B2M, which is the more numerous, self-aggregates to form oligomers and amyloid fibrils that may either protect cells against microbes or, conversely, wreak havoc in the body if the cleavage process is dysregulated (Figure 2).
References
- Cooper, J.C.; Dealtry, G.B.; Ahmed, M.A.; Arck, P.C.; Klapp, B.F.; Blois, S.M.; Fernández, N. An impaired breeding phenotype in mice with a genetic deletion of beta-2 microglobulin and diminished MHC class I expression: Role in reproductive fitness. Biol. Reprod. 2007, 77, 274–279.
- Kapadia, D.; Sadikovic, A.; Vanloubbeeck, Y.; Brockstedt, D.; Fong, L. Interplay between CD8α+ dendritic cells and monocytes in response to Listeria monocytogenes infection attenuates T cell responses. PLoS ONE 2011, 6, e19376.
- Street, S.E.; Hayakawa, Y.; Zhan, Y.; Lew, A.M.; MacGregor, D.; Jamieson, A.M.; Diefenbach, A.; Yagita, H.; Godfrey, D.I.; Smyth, M.J. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J. Exp. Med. 2004, 199, 879–884.
- Naiki, H.; Okoshi, T.; Ozawa, D.; Yamaguchi, I.; Hasegawa, K. Molecular pathogenesis of human amyloidosis: Lessons from β2 -microglobulin-related amyloidosis. Pathol. Int. 2016, 66, 193–201.
- Gejyo, F.; Yamada, T.; Odani, S.; Nakagawa, Y.; Arakawa, M.; Kunitomo, T.; Kataoka, H.; Suzuki, M.; Hirasawa, Y.; Shirahama, T.; et al. A new form of amyloid protein associated with chronic hemodialysis was identified as beta 2-microglobulin. Biochem. Biophys. Res. Commun. 1985, 129, 701–706.
- Roch, P.; Valembois, P.; Vaillier, J. Amino acid compositions and relationships of five earthworm defense proteins. Comp. Biochem. Physiol. Part B Comp. Biochem. 1986, 85, 747–751.
- Kim, J.-Y.; Park, S.-C.; Lee, J.-K.; Choi, S.J.; Hahm, K.-S.; Park, Y. Novel Antibacterial Activity of β2-Microglobulin in Human Amniotic Fluid. PLoS ONE 2012, 7, e47642.
- Roberts, A.D.; Ordway, D.J.; Orme, I.M. Listeria monocytogenes infection in beta 2 microglobulin-deficient mice. Infect. Immun. 1993, 61, 1113–1116.
- D’Souza, C.D.; Cooper, A.M.; Frank, A.A.; Ehlers, S.; Turner, J.; Bendelac, A.; Orme, I.M. A novel nonclassic beta2-microglobulin-restricted mechanism influencing early lymphocyte accumulation and subsequent resistance to tuberculosis in the lung. Am. J. Respir. Cell Mol. Biol. 2000, 23, 188–193.
- Chiou, S.J.; Wang, C.C.; Tseng, Y.S.; Lee, Y.J.; Chen, S.C.; Chou, C.H.; Chuang, L.Y.; Hong, Y.R.; Lu, C.Y.; Chiu, C.C.; et al. A novel role for β2-microglobulin: A precursor of antibacterial chemokine in respiratory epithelial cells. Sci. Rep. 2016, 6, 31035.
- Dürr, U.H.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1408–1425.
- Groß, R.; Bauer, R.; Krüger, F.; Rücker-Braun, E.; Olari, L.R.; Ständker, L.; Preising, N.; Rodríguez, A.A.; Conzelmann, C.; Gerbl, F.; et al. A Placenta Derived C-Terminal Fragment of β-Hemoglobin With Combined Antibacterial and Antiviral Activity. Front. Microbiol. 2020, 11, 508.
- Holch, A.; Bauer, R.; Olari, L.R.; Rodriguez, A.A.; Ständker, L.; Preising, N.; Karacan, M.; Wiese, S.; Walther, P.; Ruiz-Blanco, Y.B.; et al. Respiratory ß-2-Microglobulin exerts pH dependent antimicrobial activity. Virulence 2020, 11, 1402–1414.
- Bertini, R.; Howard, O.M.; Dong, H.F.; Oppenheim, J.J.; Bizzarri, C.; Sergi, R.; Caselli, G.; Pagliei, S.; Romines, B.; Wilshire, J.A.; et al. Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J. Exp. Med. 1999, 189, 1783–1789.
- Peaper, D.R.; Wearsch, P.A.; Cresswell, P. Tapasin and ERp57 form a stable disulfide-linked dimer within the MHC class I peptide-loading complex. Embo J. 2005, 24, 3613–3623.
- Wendler, J.; Schroeder, B.O.; Ehmann, D.; Koeninger, L.; Mailänder-Sánchez, D.; Lemberg, C.; Wanner, S.; Schaller, M.; Stange, E.F.; Malek, N.P.; et al. Proteolytic Degradation of reduced Human Beta Defensin 1 generates a Novel Antibiotic Octapeptide. Sci. Rep. 2019, 9, 3640.
- Mak, T.N.; Brüggemann, H. Vimentin in Bacterial Infections. Cells 2016, 5, 18.
- Lim, A.I.; Chan, L.Y.; Tang, S.C.; Yiu, W.H.; Li, R.; Lai, K.N.; Leung, J.C. BMP-7 represses albumin-induced chemokine synthesis in kidney tubular epithelial cells through destabilization of NF-κB-inducing kinase. Immunol. Cell Biol. 2014, 92, 427–435.
- Linge, H.M.; Collin, M.; Nordenfelt, P.; Mörgelin, M.; Malmsten, M.; Egesten, A. The human CXC chemokine granulocyte chemotactic protein 2 (GCP-2)/CXCL6 possesses membrane-disrupting properties and is antibacterial. Antimicrob. Agents Chemother. 2008, 52, 2599–2607.
- Chang, M.S.; McNinch, J.; Basu, R.; Simonet, S. Cloning and characterization of the human neutrophil-activating peptide (ENA-78) gene. J. Biol. Chem. 1994, 269, 25277–25282.
- Lee, M.M.; Yoon, B.-J.; Osiewicz, K.; Preston, M.; Bundy, B.; van Heeckeren, A.M.; Werb, Z.; Soloway, P.D. Tissue inhibitor of metalloproteinase 1 regulates resistance to infection. Infect. Immun. 2005, 73, 661–665.
- Clahsen, T.; Schaper, F. Interleukin-6 acts in the fashion of a classical chemokine on monocytic cells by inducing integrin activation, cell adhesion, actin polymerization, chemotaxis, and transmigration. J. Leukoc. Biol. 2008, 84, 1521–1529.
- Nguyen, L.T.; Chan, D.I.; Boszhard, L.; Zaat, S.A.J.; Vogel, H.J. Structure–function studies of chemokine-derived carboxy-terminal antimicrobial peptides. Biochim. Biophys. Acta (BBA) Biomembr. 2010, 1798, 1062–1072.
- Björstad, A.; Fu, H.; Karlsson, A.; Dahlgren, C.; Bylund, J. Interleukin-8-derived peptide has antibacterial activity. Antimicrob. Agents Chemother. 2005, 49, 3889–3895.
- Potapovich, A.I.; Pastore, S.; Kostyuk, V.A.; Lulli, D.; Mariani, V.; De Luca, C.; Dudich, E.I.; Korkina, L.G. alpha-Fetoprotein as a modulator of the pro-inflammatory response of human keratinocytes. Br. J. Pharm. 2009, 158, 1236–1247.
- Alunno, A.; Bistoni, O.; Manetti, M.; Cafaro, G.; Valentini, V.; Bartoloni, E.; Gerli, R.; Liso, A. Insulin-Like Growth Factor Binding Protein 6 in Rheumatoid Arthritis: A Possible Novel Chemotactic Factor? Front. Immunol. 2017, 8, 554.
- Liou, H.C.; Jin, Z.; Tumang, J.; Andjelic, S.; Smith, K.A.; Liou, M.L. c-Rel is crucial for lymphocyte proliferation but dispensable for T cell effector function. Int. Immunol. 1999, 11, 361–371.
- Malik, E.; Dennison, S.R.; Harris, F.; Phoenix, D.A. pH Dependent Antimicrobial Peptides and Proteins, Their Mechanisms of Action and Potential as Therapeutic Agents. Pharmaceuticals (Basel) 2016, 9, 67.
- Marquette, A.; Bechinger, B. Biophysical Investigations Elucidating the Mechanisms of Action of Antimicrobial Peptides and Their Synergism. Biomolecules 2018, 8, 18.
- Abou Alaiwa, M.H.; Reznikov, L.R.; Gansemer, N.D.; Sheets, K.A.; Horswill, A.R.; Stoltz, D.A.; Zabner, J.; Welsh, M.J. pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proc. Natl. Acad. Sci. USA 2014, 111, 18703.
- Edström Hägerwall, A.M.L.; Rydengård, V.; Fernlund, P.; Mörgelin, M.; Baumgarten, M.; Cole, A.M.; Malmsten, M.; Kragelund, B.B.; Sørensen, O.E. β-Microseminoprotein Endows Post Coital Seminal Plasma with Potent Candidacidal Activity by a Calcium- and pH-Dependent Mechanism. PLoS Pathog. 2012, 8, e1002625.
- Maisetta, G.; Vitali, A.; Scorciapino, M.A.; Rinaldi, A.C.; Petruzzelli, R.; Brancatisano, F.L.; Esin, S.; Stringaro, A.; Colone, M.; Luzi, C.; et al. pH-dependent disruption of Escherichia coli ATCC 25922 and model membranes by the human antimicrobial peptides hepcidin 20 and 25. FEBS J. 2013, 280, 2842–2854.
- Takahashi, M.; Takahashi, H.; Okakura, Y.; Ichikawa, M.; Kuda, T.; Kimura, B. Impact of pH and protein hydrophobicity on norovirus inactivation by heat-denatured lysozyme. PLoS ONE 2020, 15, e0237888.
- Baker, H.M.; Baker, E.N. Lactoferrin and Iron: Structural and dynamic aspects of binding and release. Biometals 2004, 17, 209–216.
- Michalek, M.; Gelhaus, C.; Hecht, O.; Podschun, R.; Schröder, J.M.; Leippe, M.; Grötzinger, J. The human antimicrobial protein psoriasin acts by permeabilization of bacterial membranes. Dev. Comp. Immunol. 2009, 33, 740–746.
- Hirsch, J.G. Studies of the bactericidal action of phagocytin. J. Exp. Med. 1956, 103, 613–621.
- Mohanan, G.; Nair, K.S.; Nampoothiri, K.M.; Bajaj, H. Engineering bio-mimicking functional vesicles with multiple compartments for quantifying molecular transport. Chem. Sci. 2020, 11, 4669–4679.
- Rosen, T.; Nolan, E.M. Metal Sequestration and Antimicrobial Activity of Human Calprotectin Are pH-Dependent. Biochemistry 2020, 59, 2468–2478.
- Hong, S.; Takahashi, H.; Nadres, E.T.; Mortazavian, H.; Caputo, G.A.; Younger, J.G.; Kuroda, K. A Cationic Amphiphilic Random Copolymer with pH-Responsive Activity against Methicillin-Resistant Staphylococcus aureus. PLoS ONE 2017, 12, e0169262.
- Kacprzyk, L.; Rydengård, V.; Mörgelin, M.; Davoudi, M.; Pasupuleti, M.; Malmsten, M.; Schmidtchen, A. Antimicrobial activity of histidine-rich peptides is dependent on acidic conditions. Biochim. Biophys. Acta (BBA) Biomembr. 2007, 1768, 2667–2680.
- Myhre, E.B.; Kronvall, G. Heterogeneity of nonimmune immunoglobulin Fc reactivity among gram-positive cocci: Description of three major types of receptors for human immunoglobulin G. Infect. Immun. 1977, 17, 475–482.
- Kronvall, G.; Myhre, E.B.; Björck, L.; Berggård, I. Binding of aggregated human beta2-microglobulin to surface protein structure in group A, C, and G streptococci. Infect. Immun. 1978, 22, 136–142.
- Mukherjee, S.; Zheng, H.; Derebe, M.G.; Callenberg, K.M.; Partch, C.L.; Rollins, D.; Propheter, D.C.; Rizo, J.; Grabe, M.; Jiang, Q.X.; et al. Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature 2014, 505, 103–107.
- Heegaard, N.H. beta(2)-microglobulin: From physiology to amyloidosis. Amyloid 2009, 16, 151–173.
- Karlsson, F.A.; Wibell, L.; Evrin, P.E. beta 2-Microglobulin in clinical medicine. Scand. J. Clin. Lab. Invest. Suppl. 1980, 154, 27–37.
- Hetz, C.; Bono, M.R.; Barros, L.F.; Lagos, R. Microcin E492, a channel-forming bacteriocin from Klebsiella pneumoniae, induces apoptosis in some human cell lines. Proc. Natl. Acad. Sci. USA 2002, 99, 2696–2701.
