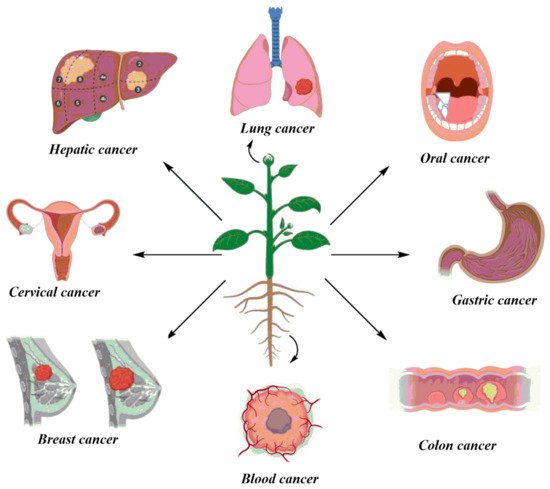
The rising burden of cancer worldwide calls for an alternative treatment solution. Herbal medicine provides a very feasible alternative to western medicine against cancer. This aentrticley reviews the selected plant species with active phytochemicals, the animal models used for these studies, and their regulatory aspects. This study is based on a meticulous literature review conducted through the search of relevant keywords in databases, Web of Science, Scopus, PubMed, and Google Scholar. Twenty plants were selected based on defined selection criteria for their potent anticancer compounds. The detailed analysis of the research studies revealed that plants play an indispensable role in fighting different cancers such as breast, stomach, oral, colon, lung, hepatic, cervical, and blood cancer cell lines. The in vitro studies showed cancer cell inhibition through DNA damage and activation of apoptosis-inducing enzymes by the secondary metabolites in the plant extracts. Studies that reported in vivo activities of these plants showed remarkable results in the inhibition of cancer in animal models.
- cancer
- apoptosis
- herbs
- cell lines
- in vivo
1. Introduction
2. Selected Plants and Their Anticancer Activity
Research so far has tested the anticancer activity of a plethora of plants and plant-based compounds. Some of these plants and their compounds prove to be very effective against one or more types of cancers. Based on their activities, the following plants are selected for the in vitro and in vivo anticancer activities of their compounds. The rest of the important plants shortlisted for their activities are presented in Table 1 along with their activities.2.1. Artemisia annua
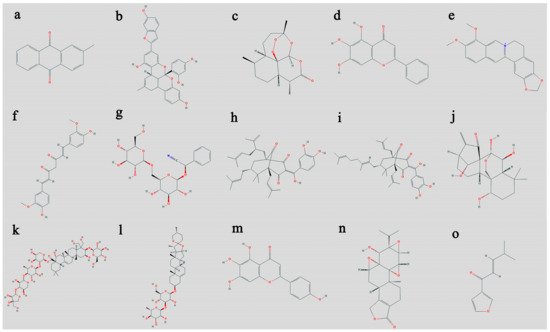
2.2. Coptis chinensis
2.3. Curcuma longa
2.4. Fagonia indica
2.5. Garcinia oblongifolia
2.6. Garcinia indica
2.7. Hedyotis diffusa
3. In Vivo Studies of Anticancer Herbal Medicine: An Overview
The herbal medicines are tested both in vitro and in vivo. The anticancer activities of the various medicinal plants have been tested in vivo using different animal models (Figure 3). There are many studies available on in vivo experiments of the many different anticancer plants in mice models. For instance, dihydroartemisinin was reported to inhibit tumor tissue, increase the level of interferon-gamma (IFN-γ), and decrease interleukin 4 (IL-4) in tumor-bearing mice [154][60]. Similarly, artesunate, a derivative of artemisinin is also reported to be a promising drug against angiogenic Kaposi′s sarcoma [155][61], growth inhibition of A549 and H1299 lung tumors by 100 mg/kg dose [156][62], the suppression of human prostate cancer xenograft [157][63] and the inhibition of leukemia growth in mice [158][64].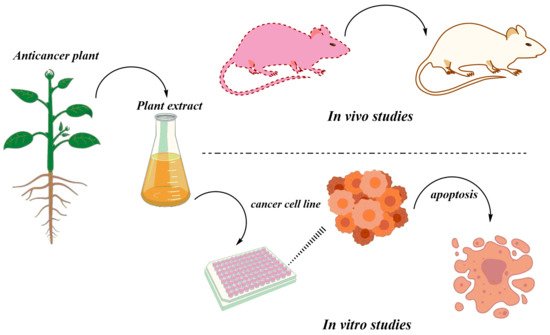
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Karpuz, M.; Silindir-Gunay, M.; Ozer, A.Y. Current and Future Approaches for Effective Cancer Imaging and Treatment. Cancer Biother. Radiopharm. 2018, 33, 39–51.
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural compounds for cancer treatment and prevention. Pharmacol. Res. 2009, 59, 365–378.
- Cheng, H. Advanced Textbook on Traditional Chinese Medicine and Pharmacology; New World Press: Beijing, China, 1995.
- Shehab, N.G.; Mahdy, A.; Khan, S.A.; Noureddin, S.M. Chemical constituents and biological activities of Fagonia indica Burm F. Res. J. Med. Plant. 2011, 5, 531–546.
- Faried, A.; Kurnia, D.; Faried, L.; Usman, N.; Miyazaki, T.; Kato, H.; Kuwano, H. Anticancer effects of gallic acid isolated from Indonesian herbal medicine, Phaleria macrocarpa (Scheff.) Boerl, on human cancer cell lines. Int. J. Oncol. 2007, 30, 605–613.
- Sohi, K.K.; Mittal, N.; Hundal, M.K.; Khanduja, K.L. Gallic acid, an antioxidant, exhibits antiapoptotic potential in normal human lymphocytes: A Bcl-2 independent mechanism. J. Nutr. Sci. Vitaminol. (Tokyo) 2003, 49, 221–227.
- Inoue, M.; Suzuki, R.; Koide, T.; Sakaguchi, N.; Ogihara, Y.; Yabu, Y. Antioxidant, gallic acid, induces apoptosis in HL-60RG cells. Biochem. Biophys. Res. Commun. 1994, 204, 898–904.
- Sun, J.; Chu, Y.-F.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002, 50, 7449–7454.
- Taraphdar, A.K.; Roy, M.; Bhattacharya, R. Natural products as inducers of apoptosis: Implication for cancer therapy and prevention. Curr. Sci. 2001, 1387–1396.
- Pal, S.K.; Shukla, Y. Herbal medicine: Current status and the future. Asian Pac. J. Cancer Prev. 2003, 4, 281–288.
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220.
- Saklani, A.; Kutty, S.K. Plant-derived compounds in clinical trials. Drug Discov. Today 2008, 13, 161–171.
- Wang, C.-Z.; Calway, T.; Yuan, C.-S. Herbal medicines as adjuvants for cancer therapeutics. Am. J. Chin. Med. 2012, 40, 657–669.
- Fattovich, G.; Stroffolini, T.; Zagni, I.; Donato, F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 2004, 127, S35–S50.
- Llovet, J.M. Updated treatment approach to hepatocellular carcinoma. J. Gastroenterol. 2005, 40, 225–235.
- Ruan, W.-J.; Lai, M.-D.; Zhou, J.-G. Anticancer effects of Chinese herbal medicine, science or myth? J. Zhejiang Univ. Sci. B 2006, 7, 1006–1014.
- Trease, G.; Evans, W. Textbook of Pharmacognosy; Balliere: London, UK, 1983; pp. 57–59.
- Ghorani-Azam, A.; Sepahi, S.; Riahi-Zanjani, B.; Ghamsari, A.A.; Mohajeri, S.A.; Balali-Mood, M. Plant toxins and acute medicinal plant poisoning in children: A systematic literature review. J. Res. Med. Sci. 2018, 23, 26.
- Abad, M.J.; Bedoya, L.M.; Bermejo, P. Essential Oils from the Asteraceae Family Active against Multidrug-Resistant Bacteria. In Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components; Rai, M.K., Kon, K.V., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 205–221.
- Tan, R.X.; Zheng, W.; Tang, H. Biologically active substances from the genus Artemisia. Planta Med. 1998, 64, 295–302.
- Lu, H.; Zou, W.X.; Meng, J.C.; Hu, J.; Tan, R.X. New bioactive metabolites produced by Colletotrichum sp., an endophytic fungus in Artemisia annua. Plant. Sci. 2000, 151, 67–73.
- Efferth, T. Mechanistic perspectives for 1, 2, 4-trioxanes in anti-cancer therapy. Drug Resist. Updat. 2005, 8, 85–97.
- Ryu, J.-H.; Lee, S.-J.; Kim, M.-J.; Shin, J.-H.; Kang, S.-K.; Cho, K.-M.; Sung, N.-J. Antioxidant and anticancer activities of Artemisia annua L. and determination of functional compounds. J. Korean Soc. Food Sci. Nutr. 2011, 40, 509–516.
- Xie, W.; Gu, D.; Li, J.; Cui, K.; Zhang, Y. Effects and action mechanisms of berberine and Rhizoma coptidis on gut microbes and obesity in high-fat diet-fed C57BL/6J mice. PLoS ONE 2011, 6, e24520.
- Yin, J.; Zhang, H.; Ye, J. Traditional Chinese medicine in treatment of metabolic syndrome. Endocr. Metab. Immune Disord. Drug Targets 2008, 8, 99–111.
- Hu, Y.; Davies, G.E. Berberine inhibits adipogenesis in high-fat diet-induced obesity mice. Fitoterapia 2010, 81, 358–366.
- Tang, J.; Feng, Y.; Tsao, S.; Wang, N.; Curtain, R.; Wang, Y. Berberine and Coptidis rhizoma as novel antineoplastic agents: A review of traditional use and biomedical investigations. J. Ethnopharmacol. 2009, 126, 5–17.
- Ammon, H.P.; Wahl, M.A. Pharmacology of Curcuma longa. Planta Med. 1991, 57, 1–7.
- Liu, F.; Ng, T. Antioxidative and free radical scavenging activities of selected medicinal herbs. Life Sci. 2000, 66, 725–735.
- Schinella, G.; Tournier, H.; Prieto, J.; De Buschiazzo, P.M.; Rıos, J. Antioxidant activity of anti-inflammatory plant extracts. Life Sci. 2002, 70, 1023–1033.
- Goel, A.; Boland, C.R.; Chauhan, D.P. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001, 172, 111–118.
- Wang, X.; Hang, Y.; Liu, J.; Hou, Y.; Wang, N.; Wang, M. Anticancer effect of curcumin inhibits cell growth through miR-21/PTEN/Akt pathway in breast cancer cell. Oncol. Lett. 2017, 13, 4825–4831.
- Beier, B.A. A revision of the desert shrub Fagonia (Zygophyllaceae). Syst. Biodivers. 2005, 3, 221–263.
- Akhtar, N.; Begum, S. Ethnopharmacological important plants of Jalala, district Mardan, Pakistan. Pak. J. Pl. Sci. 2009, 15, 95–100.
- Sharrma, S.; Gupta, V.; Sharma, G. Phytopharmacology of Fagonia indica (L): A review. J. Nat. Cons. 2010, 1, 143–147.
- Ibrahim, L.F.; Kawashty, S.A.; El-Hagrassy, A.M.; Nassar, M.I.; Mabry, T.J. A new kaempferol triglycoside from Fagonia taeckholmiana: Cytotoxic activity of its extracts. Carbohydr. Res. 2008, 343, 155–158.
- Sharawy, S.M.; Alshammari, A.M. Checklist of poisonous plants and animals in Aja Mountain, Ha’il Region, Saudi Arabia. Aust. J. Basic Appl. Sci. 2009, 3, 2217–2225.
- Shaker, K.H.; Bernhardt, M.; Elgamal, M.H.A.; Seifert, K. Triterpenoid saponins from Fagonia indica. Phytochemistry 1999, 51, 1049–1053.
- Perrone, A.; Masullo, M.; Bassarello, C.; Hamed, A.I.; Belisario, M.A.; Pizza, C.; Piacente, S. Sulfated triterpene derivatives from Fagonia arabica. J. Nat. Prod. 2007, 70, 584–588.
- Bagban, I.; Roy, S.; Chaudhary, A.; Das, S.; Gohil, K.; Bhandari, K. Hepatoprotective activity of the methanolic extract of Fagonia indica Burm in carbon tetra chloride induced hepatotoxicity in albino rats. Asian Pac. J. Trop. Biomed. 2012, 2, S1457–S1460.
- Eman, A.A. Morphological, phytochemical and biological screening on three Egyptian species of Fagonia. Acad Arena 2011, 3, 18–27.
- Waheed, A.; Barker, J.; Barton, S.J.; Owen, C.P.; Ahmed, S.; Carew, M.A. A novel steroidal saponin glycoside from Fagonia indica induces cell-selective apoptosis or necrosis in cancer cells. Eur. J. Pharm. Sci. 2012, 47, 464–473.
- Lam, M.; Carmichael, A.R.; Griffiths, H.R. An aqueous extract of Fagonia cretica induces DNA damage, cell cycle arrest and apoptosis in breast cancer cells via FOXO3a and p53 expression. PLoS ONE 2012, 7, e40152.
- Wu, S.-B.; Long, C.; Kennelly, E.J. Structural diversity and bioactivities of natural benzophenones. Nat. Prod. Rep. 2014, 31, 1158–1174.
- Hemshekhar, M.; Sunitha, K.; Santhosh, M.S.; Devaraja, S.; Kemparaju, K.; Vishwanath, B.; Niranjana, S.; Girish, K. An overview on genus Garcinia: Phytochemical and therapeutical aspects. Phytochem. Rev. 2011, 10, 325–351.
- Huang, S.-X.; Feng, C.; Zhou, Y.; Xu, G.; Han, Q.-B.; Qiao, C.-F.; Chang, D.C.; Luo, K.Q.; Xu, H.-X. Bioassay-guided isolation of xanthones and polycyclic prenylated acylphloroglucinols from Garcinia oblongifolia. J. Nat. Prod. 2008, 72, 130–135.
- Feng, C.; Huang, S.-X.; Gao, X.-M.; Xu, H.-X.; Luo, K.Q. Characterization of Proapoptotic Compounds from the Bark of Garcinia oblongifolia. J. Nat. Prod. 2014, 77, 1111–1116.
- Li, P.; AnandhiSenthilkumar, H.; Wu, S.-B.; Liu, B.; Guo, Z.-Y.; Fata, J.E.; Kennelly, E.J.; Long, C.-L. Comparative UPLC-QTOF-MS-based metabolomics and bioactivities analyses of Garcinia oblongifolia. J. Chromatogr. B 2016, 1011, 179–195.
- Hong, J.; Kwon, S.J.; Sang, S.; Ju, J.; Zhou, J.-N.; Ho, C.-T.; Huang, M.-T.; Yang, C.S. Effects of garcinol and its derivatives on intestinal cell growth: Inhibitory effects and autoxidation-dependent growth-stimulatory effects. Free Radic. Biol. Med. 2007, 42, 1211–1221.
- Liao, C.H.; Sang, S.; Ho, C.T.; Lin, J.K. Garcinol modulates tyrosine phosphorylation of FAK and subsequently induces apoptosis through down-regulation of Src, ERK, and Akt survival signaling in human colon cancer cells. J. Cell. Biochem. 2005, 96, 155–169.
- Pan, M.-H.; Chang, W.-L.; Lin-Shiau, S.-Y.; Ho, C.-T.; Lin, J.-K. Induction of apoptosis by garcinol and curcumin through cytochrome c release and activation of caspases in human leukemia HL-60 cells. J. Agric. Food Chem. 2001, 49, 1464–1474.
- Lin, C.-C.; Ng, L.-T.; Yang, J.-J.; Hsu, Y.-F. Anti-inflammatory and hepatoprotective activity of peh-hue-juwa-chi-cao in male rats. Am. J. Chin. Med. 2002, 30, 225–234.
- Ahmad, R.; Ali, A.M.; Israf, D.A.; Ismail, N.H.; Shaari, K.; Lajis, N.H. Antioxidant, radical-scavenging, anti-inflammatory, cytotoxic and antibacterial activities of methanolic extracts of some Hedyotis species. Life Sci. 2005, 76, 1953–1964.
- Fang, Y.; Zhang, Y.; Chen, M.; Zheng, H.; Zhang, K. The active component of Hedyotis diffusa Willd. Chin. Tradit. Plant. Med. 2004, 26, 577–579.
- Ahmad, R.; Shaari, K.; Lajis, N.H.; Hamzah, A.S.; Ismail, N.H.; Kitajima, M. Anthraquinones from Hedyotis capitellata. Phytochemistry 2005, 66, 1141–1147.
- Li, C.; Xue, X.; Zhou, D.; Zhang, F.; Xu, Q.; Ren, L.; Liang, X. Analysis of iridoid glucosides in Hedyotis diffusa by high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2008, 48, 205–211.
- Liu, Z.; Liu, M.; Liu, M.; Li, J. Methylanthraquinone from Hedyotis diffusa WILLD induces Ca2+-mediated apoptosis in human breast cancer cells. Toxicol. Vitr. 2010, 24, 142–147.
- Wu, Z.; Jin, C.; Li, J.; Chen, X.; Yao, Q.; Zhu, Q. Inhibition of Colon Cancer Cells by Ethanol Extract of Oldenlandia diffusa. J. Kunming Med. Univ. 2013, 10, 31–34.
- Noori, S.; Hassan, Z.M. Dihydroartemisinin shift the immune response towards Th1, inhibit the tumor growth in vitro and in vivo. Cell. Immunol. 2011, 271, 67–72.
- Dell’Eva, R.; Pfeffer, U.; Vené, R.; Anfosso, L.; Forlani, A.; Albini, A.; Efferth, T. Inhibition of angiogenesis in vivo and growth of Kaposi’s sarcoma xenograft tumors by the anti-malarial artesunate. Biochem. Pharmacol. 2004, 68, 2359–2366.
- Katiyar, S.K.; Meeran, S.M.; Katiyar, N.; Akhtar, S. p53 cooperates berberine-induced growth inhibition and apoptosis of non-small cell human lung cancer cells in vitro and tumor xenograft growth in vivo. Mol. Carcinog. 2009, 48, 24–37.
- Choi, M.S.; Oh, J.H.; Kim, S.M.; Jung, H.Y.; Yoo, H.S.; Lee, Y.M.; Moon, D.C.; Han, S.B.; Hong, J.T. Berberine inhibits p53-dependent cell growth through induction of apoptosis of prostate cancer cells. Int. J. Oncol. 2009, 34, 1221–1230.
- Harikumar, K.B.; Kuttan, G.; Kuttan, R. Inhibition of progression of erythroleukemia induced by Friend virus in BALB/c mice by natural products—Berberine, Curcumin and Picroliv. J. Exp. Ther. Oncol. 2008, 7, 275–284.
- Peng, P.-L.; Kuo, W.-H.; Tseng, H.-C.; Chou, F.-P. Synergistic Tumor-Killing Effect of Radiation and Berberine Combined Treatment in Lung Cancer: The Contribution of Autophagic Cell Death. Int. J. Radiat. Oncol. 2008, 70, 529–542.
- Huang, T.; Xiao, Y.; Yi, L.; Li, L.; Wang, M.; Tian, C.; Ma, H.; He, K.; Wang, Y.; Han, B.; et al. Coptisine from Rhizoma coptidis Suppresses HCT-116 Cells-related Tumor Growth in vitro and in vivo. Sci. Rep. 2017, 7, 38524.
- Chen, X.-Z.; Cao, Z.-Y.; Chen, T.-S.; Zhang, Y.-Q.; Liu, Z.-Z.; Su, Y.-T.; Liao, L.-M.; Du, J. Water extract of Hedyotis diffusa Willd suppresses proliferation of human HepG2 cells and potentiates the anticancer efficacy of low-dose 5-fluorouracil by inhibiting the CDK2-E2F1 pathway. Oncol. Rep. 2012, 28, 742–748.
- Yang, X.; Yang, Y.; Tang, S.; Tang, H.; Yang, G.; Xu, Q.; Wu, J. Anti-tumor effect of polysaccharides from Scutellaria barbata D. Don on the 95-D xenograft model via inhibition of the C-met pathway. J. Pharmacol. Sci. 2014, 125, 255–263.
- Li, Y.; Gu, J.-F.; Zou, X.; Wu, J.; Zhang, M.-H.; Jiang, J.; Qin, D.; Zhou, J.-Y.; Liu, B.-X.-Z.; Zhu, Y.-T. The anti-lung cancer activities of steroidal saponins of P. polyphylla Smith var. chinensis (Franch.) Hara through enhanced immunostimulation in experimental Lewis tumor-bearing C57BL/6 mice and induction of apoptosis in the A549 cell line. Molecules 2013, 18, 12916–12936.
- Wanga, Y.; Huangb, X.; Hanc, J.; Zhenga, W.; Maa, W. Extract of Perilla frutescens inhibits tumor proliferation of HCC via PI3K/AKT signal pathway. Afr. J. Tradit. Complementary Altern. Med. 2013, 10, 251–257.
- Fukutake, M.; Yokota, S.; Kawamura, H.; Iizuka, A.; Amagaya, S.; Fukuda, K.; Komatsu, Y. Inhibitory Effect of Coptidis rhizoma and Scutellariae Radix on Azoxymethane-Induced Aberrant Crypt Foci Formation in Rat Colon. Biol. Pharm. Bull. 1998, 21, 814–817.
- Manjamalai, A.; Grace, B. Chemotherapeutic effect of essential oil of Wedelia chinensis (Osbeck) on inducing apoptosis, suppressing angiogenesis and lung metastasis in C57BL/6 mice model. J. Cancer Sci. Ther. 2013, 5, 271–281.
- Bao, R.; Shu, Y.; Wu, X.; Weng, H.; Ding, Q.; Cao, Y.; Li, M.; Mu, J.; Wu, W.; Ding, Q. Oridonin induces apoptosis and cell cycle arrest of gallbladder cancer cells via the mitochondrial pathway. BMC Cancer 2014, 14, 217.
- Singh, N.P.; Lai, H.C.; Park, J.S.; Gerhardt, T.E.; Kim, B.J.; Wang, S.; Sasaki, T. Effects of artemisinin dimers on rat breast cancer cells in vitro and in vivo. Anticancer Res. 2011, 31, 4111–4114.
- Lai, H.; Singh, N.P. Oral artemisinin prevents and delays the development of 7, 12-dimethylbenz [a] anthracene (DMBA)-induced breast cancer in the rat. Cancer Lett. 2006, 231, 43–48.
- Joe, B.; Rao, U.J.; Lokesh, B.R. Presence of an acidic glycoprotein in the serum of arthritic rats: Modulation by capsaicin and curcumin. Mol. Cell. Biochem. 1997, 169, 125–134.
- Tanaka, T.; Kohno, H.; Shimada, R.; Kagami, S.; Yamaguchi, F.; Kataoka, S.; Ariga, T.; Murakami, A.; Koshimizu, K.; Ohigashi, H. Prevention of colonic aberrant crypt foci by dietary feeding of garcinol in male F344 rats. Carcinogenesis 2000, 21, 1183–1189.
- Liu, S.; Leach, S.D. Zebrafish models for cancer. Annu. Rev. Pathol. 2011, 6, 71–93.
- Zhu, X.-Y.; Guo, D.-W.; Lao, Q.-C.; Xu, Y.-Q.; Meng, Z.-K.; Xia, B.; Yang, H.; Li, C.-Q.; Li, P. Sensitization and synergistic anti-cancer effects of Furanodiene identified in zebrafish models. Sci. Rep. 2019, 9, 4541.
- Efferth, T.; Dunstan, H.; Sauerbrey, A.; Miyachi, H.; Chitambar, C.R. The anti-malarial artesunate is also active against cancer. Int. J. Oncol. 2001, 18, 767–773.
- Efferth, T. Molecular pharmacology and pharmacogenomics of artemisinin and its derivatives in cancer cells. Curr. Drug Targets 2006, 7, 407–421.
- Breuer, E.; Efferth, T. Treatment of Iron-Loaded Veterinary Sarcoma by Artemisia annua. Nat. Prod. Bioprospecting 2014, 4, 113–118.
