Fertility preservation is an emerging discipline, which is of substantial clinical value in the care of young patients with cancer. Chemotherapy and radiation may induce ovarian damage in prepubertal girls and young women. Although many studies have explored the mechanisms implicated in ovarian toxicity during cancer treatment, its molecular pathophysiology is not fully understood.
- chemotherapy
- radiotherapy
- gonadotoxicity
- fertility preservation
- embryo cryopreservation
- oocyte cryopreservation
- ovarian tissue cryopreservation
- oocyte in vitro maturation
- ovarian suppression
- oncofertility
1. Introduction
It is estimated that more than 9.2 million women were newly diagnosed with cancer worldwide in 2020 [1]. Furthermore, there were 89,500 new cancer cases and 9270 cancer deaths in adolescents and young adults (AYAs) aged 15–39 years in the United States [2]. The survival of cancer patients has significantly improved due to recent advances in cancer treatment [3,4][3][4]. However, oncologic therapies can affect ovarian function in young women [5,6,7,8][5][6][7][8]. The exhaustion of ovarian follicle reservoirs may lead to not only loss of fertility but also premature ovarian failure, which could result in poor quality of life in young female cancer survivors [9,10,11][9][10][11]. Recently, fertility preservation (FP) has become an emerging discipline with significant clinical value in the care of AYA cancer patients [12[12][13][14],13,14], and many organizations have provided recommendations for FP during cancer treatment [15,16,17,18,19][15][16][17][18][19].
Chemotherapy has toxic effects on the ovaries and causes the loss of the primordial follicle (PF) reserve [20]. Endocrine therapy can increase the risk of infertility in patients with hormone receptor-positive malignancies [21]. In the case of abdominal or pelvic cancers, treatments including radiotherapy or surgery may alter future fertility because of direct gonadal damage [22,23][22][23]. Many studies have explored the mechanisms implicated in ovarian toxicity during cancer treatment; however, the underlying molecular pathophysiology is not fully understood [24,25,26,27,28][24][25][26][27][28].
This article will review the mechanisms of cancer therapy-induced ovarian dysfunction and explore the future perspectives for preventing infertility in AYAs with cancer.
2. Cancer Treatment-Induced Ovarian Damage
Ionizing radiation to the abdominopelvic region has deleterious effects on gonadal function at all ages [70][29]. For example, cervical and rectal cancers usually require pelvic irradiation, and craniospinal radiotherapy is performed in cases of central nervous system malignancy. In some patients with Hodgkin’s disease, pelvic lymph nodes require irradiation, and total body irradiation may be necessary prior to bone marrow transplantation.
The resulting damage depends on the dose and field of irradiation and the age of the patient. Women who received radiation treatment outside the pelvis had a low risk of ovarian dysfunction [71][30]. In the prepubertal period, the ovaries are relatively resistant to gonadotoxicity [72][31].
Dividing GCs appear to be the main target of radiation-related gonadotoxicity. Prominent cell death has been observed within a few hours of irradiation [73][32]. Oocytes are highly radiosensitive because the estimated dose at which half of the follicles are lost in humans (LD50) is <2 Gy [74][33]. A single oocyte is highly radiosensitive to a D 0 of 0.12 Gy (reciprocal of the slope of the exponential region of a survival curve). This sensitivity is affected by age; women younger than 40 years of age are less sensitive, requiring 20 Gy to experience permanent damage, whereas older women require only 6 Gy [75][34]. The radiosensitivity of oocytes differs according to their growth phase. A quiescent PF is usually more radio-resistant than a large maturing follicle [74][33]. Radiotherapy-induced ovarian damage also occurs in the stroma with vascular damage, resulting in tissue atrophy and fibrosis [73][32]. In general, a combination of multiple factors determines the extent of radiosensitivity, including age, the use of combination therapy, and radiation dose [76][35].
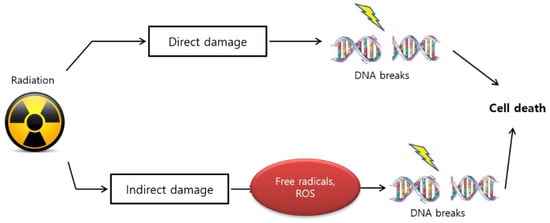
The biological effect of radiation treatment is also affected by linear energy transfer (LET) in tumors [77][36]. LET radiation induces anticancer effects by depositing physical energy or radiation into malignant cells, which results in stable free radicals and induces cellular damage because of the direct ionization of the cellular macromolecules, such as DNA, RNA, lipids, and proteins [78][37]. High LET radiation results in gonadal DNA damage that causes multiple lesions within the helical turns of the DNA, which is referred to as “direct” damage ( Figure 31 ).
3. Detection of Ovarian Damage
However, the level of AMH does not always correlate with the quality of oocytes because it only reflects the quantity of oocytes [97][38]. Additionally, AMH concentration could be altered by the handling of the blood sample or the assay method used to measure AMH levels [98][39].
After chemotherapy, FSH levels usually increase due to follicular depletion. However, basal FSH is not always a valuable marker of ovarian reserve in patients who have undergone cancer treatment. For example, if women have regular menstrual cycles, FSH levels may show normal values, even though the ovarian reserve decreases after treatment [89,99][40][41]. In such instances, that is, when the FSH levels are within the normal range, estradiol concentration in the early follicular phase may provide additional information [100][42].
Inhibin-B is secreted by the GCs of antral follicles and it regulates FSH levels via a negative feedback reaction. Inhibin-B is usually exhibited at low levels in women with a decreased ovarian reserve [101][43]. However, it is not a reliable marker of the ovarian reserve because its levels vary widely during menstrual cycles [102][44].
During the early follicular phase, transvaginal ultrasound can be used to count antral follicles measuring 2–10 mm in both ovaries [103][45]. A low AFC may be related to a diminished response to ovarian stimulation. Furthermore, a few studies have demonstrated that a low AFC could be a marker for the risk of developing amenorrhea after cancer treatment [94,96][46][47]. However, the estimation of ovarian volume using ultrasound provides limited clinical utility as an ovarian reserve marker.
4. Prevention and Management of Ovarian Damage
As oocyte freezing involves the removal of cumulus cells before cryopreservation, it can induce changes in the zona pellucida, which may affect the fertilization rates of conventional insemination. Therefore, ASRM recommends intracytoplasmic sperm injection for frozen oocytes as the preferred procedure [168,192][48][49].
The combination of oocyte cryopreservation and ovarian tissue cryopreservation can enhance the results of the FP procedure [193][50]. However, the cryopreservation of ovarian tissue concomitant with oocyte retrieval is ineffective; thus, it is not recommended after ovarian stimulation with human menopausal gonadotropin or recombinant FSH followed by human chorionic gonadotropin [194,195][51][52].
Ovarian tissue cryopreservation is generally the only option for FP in children or AYAs with cancer who need immediate treatment and do not have enough time for ovarian stimulation and other procedures. Using this technique, a large number of oocytes, including PFs, can be preserved, and hormonal function of the ovary can be protected to improve the quality of life of the young patients [196][53].
Oocyte cryopreservation is not suitable for patients with ovarian or hematologic malignancies because of the possible contamination of the ovarian tissue with malignant cells, as shown in several studies [202,203][54][55]. Nonetheless, ovarian tissue cryopreservation may be considered after an initial dose of chemotherapy to reduce the risk of malignant cell contamination, despite possible partial ovarian damage [204][56].
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Miller, K.D.; Benaoudia, M.F.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults. CA Cancer J. Clin. 2020, 70, 443–459.
- Birch, J.M.; Marsden, H.B.; Jones, P.H.; Pearson, D.; Blair, V. Improvements in survival from childhood cancer: Results of a population based survey over 30 years. Br. Med. J. (Clin. Res. Ed.) 1988, 296, 1372–1376.
- Wingo, P.A.; Ries, L.A.; Parker, S.L.; Heath, C.W., Jr. Long-term cancer patient survival in the United States. Cancer Epidemiol. Biomark. Prev. 1998, 7, 271–282.
- Sklar, C.A.; Mertens, A.C.; Mitby, P.; Whitton, J.; Stovall, M.; Kasper, C.; Mulder, J.; Green, D.; Nicholson, H.S.; Yasui, Y.; et al. Premature menopause in survivors of childhood cancer: A report from the childhood cancer survivor study. J. Natl. Cancer Inst. 2006, 98, 890–896.
- Wallace, W.H.B.; Thomson, A.B.; Saran, F.; Kelsey, T.W. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 738–744.
- Vriens, I.J.H.; de Bie, A.J.R.; Aarts, M.J.B.; de Boer, M.; van Hellemond, I.E.G.; Roijen, J.H.E.; van Golde, R.J.T.; Voogd, A.C.; Heijnen, V.C.G.T. The correlation of age with chemotherapy-induced ovarian function failure in breast cancer patients. Oncotarget 2017, 8, 11372–11379.
- Chemaitilly, W.; Li, Z.; Krasin, M.J.; Brooke, R.J.; Wilson, C.L.; Green, D.M.; Klosky, J.L.; Barnes, N.; Clark, K.L.; Farr, J.B.; et al. Premature Ovarian Insufficiency in Childhood Cancer Survivors: A Report From the St. Jude Lifetime Cohort. J. Clin. Endocrinol. Metab. 2017, 102, 2242–2250.
- Ganz, P.A.; Greendale, G.A.; Petersen, L.; Kahn, B.; Bower, J.E. Breast cancer in younger women: Reproductive and late health effects of treatment. J. Clin. Oncol. 2003, 21, 4184–4193.
- Muka, T.; Williams, C.O.; Kunutsor, S.; Laven, J.S.E.; Fauser, B.C.J.M.; Chowdhury, R.; Kavousi, M.; Franco, O.H. Association of Age at Onset of Menopause and Time Since Onset of Menopause With Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality: A Systematic Review and Meta-analysis. JAMA Cardiol. 2016, 1, 767–776.
- Wu, X.; Cai, H.; Kallianpur, A.; Li, H.; Yang, G.; Gao, J.; Xiang, Y.B.; Ji, B.T.; Tang, Y.; Zheng, W.; et al. Impact of premature ovarian failure on mortality and morbidity among Chinese women. PLoS ONE 2014, 9, e89597.
- Lee, S.; Heytens, E.; Moy, F.; Ozkavukcu, S.; Oktay, K. Determinants of access to fertility preservation in women with breast cancer. Fertil. Steril. 2011, 95, 1932–1936.
- Lee, S.; Ozkavukcu, S.; Heytens, E.; Moy, F.; Oktay, K. Value of early referral to fertility preservation in young women with breast cancer. J. Clin. Oncol. 2010, 28, 4683–4686.
- Kim, J.; Oktay, K.; Gracia, C.; Lee, S.; Morse, C.; Mersereau, J.E. Which patients pursue fertility preservation treatments? A multicenter analysis of the predictors of fertility preservation in women with breast cancer. Fertil. Steril. 2012, 97, 671–676.
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001.
- Lee, S.; Kim, S.K.; Hwang, K.J.; Kim, T.; Kim, S.H. Fertility preservation for patients with gynecologic malignancies: The Korean Society for Fertility Preservation clinical guidelines. Clin. Exp. Reprod. Med. 2017, 44, 175–180.
- Campbell, S.B.; Woodard, T.L. An update on fertility preservation strategies for women with cancer. Gynecol. Oncol. 2020, 156, 3–5.
- Lambertini, M.; Peccatori, F.A.; Demeestere, I.; Amant, F.; Wyns, C.; Stukenborg, J.-B.; Paluch-Shimon, S.; Halaska, M.J.; Uzan, C.; Meissner, J.; et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines(dagger). Ann. Oncol. 2020, 31, 1664–1678.
- Coccia, P.F.; Pappo, A.S.; Beaupin, L.; Borges, V.F.; Borinstein, S.C.; Chugh, R.; Dinner, S.; Folbrecht, J.; Frazier, A.L.; Goldsby, R.; et al. Adolescent and Young Adult Oncology, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2018, 16, 66–97.
- Letourneau, J.M.; Ebbel, E.E.; Katz, P.P.; Oktay, K.H.; McCulloch, C.E.; Ai, W.Z.; Chien, A.J.; Melisko, M.E.; Cedars, M.I.; Rosen, M.P. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer 2012, 118, 1933–1939.
- Davies, C.; Pan, H.; Godwin, J.; Gray, R.; Arriagada, R.; Raina, V.; Abraham, M.; Alencar, V.H.M.; Badran, A.; Bonfill, X.; et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013, 381, 805–816.
- Stupart, D.; Win, A.K.; Jenkins, M.; Winship, I.M. Female fertility and colorectal cancer. Int. J. Colorectal. Dis. 2008, 23, 735–743.
- Zapardiel, I.; Cruz, M.; Diestro, M.D.; Requena, A.; Velasco, J.A.G. Assisted reproductive techniques after fertility-sparing treatments in gynaecological cancers. Hum. Reprod. Update 2016, 22, 281–305.
- Roness, H.; Kashi, O.; Meirow, D. Prevention of chemotherapy-induced ovarian damage. Fertil. Steril. 2016, 105, 20–29.
- Sonigo, C.; Beau, I.; Binart, N.; Grynberg, M. The Impact of Chemotherapy on the Ovaries: Molecular Aspects and the Prevention of Ovarian Damage. Int. J. Mol. Sci. 2019, 20, 5342.
- Szymanska, K.J.; Tan, X.; Oktay, K. Unraveling the mechanisms of chemotherapy-induced damage to human primordial follicle reserve: Road to developing therapeutics for fertility preservation and reversing ovarian aging. Mol. Hum. Reprod. 2020, 26, 553–566.
- Cosgrove, C.M.; Salani, R. Ovarian effects of radiation and cytotoxic chemotherapy damage. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 55, 37–48.
- Marci, R.; Mallozzi, M.; di Benedetto, L.; Schimberni, M.; Mossa, S.; Soave, I.; Palomba, S.; Caserta, D. Radiations and female fertility. Reprod. Biol. Endocrinol. 2018, 16, 112.
- Howell, S.; Shalet, S. Gonadal damage from chemotherapy and radiotherapy. Endocrinol. Metab. Clin. N. Am. 1998, 27, 927–943.
- Madsen, B.L.; Giudice, L.; Donaldson, S.S. Radiation-induced premature menopause: A misconception. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 1461–1464.
- Beerendonk, C.C.M.; Braat, D.D.M. Present and future options for the preservation of fertility in female adolescents with cancer. Endocr. Dev. 2005, 8, 166–175.
- Stroud, J.S.; Mutch, D.; Rader, J.; Powell, M.; Thaker, P.H.; Grigsby, P.W. Effects of cancer treatment on ovarian function. Fertil. Steril. 2009, 92, 417–427.
- Wallace, W.H.; Thomson, A.B.; Kelsey, T.W. The radiosensitivity of the human oocyte. Hum. Reprod. 2003, 18, 117–121.
- Lushbaugh, C.C.; Casarett, G.W. The effects of gonadal irradiation in clinical radiation therapy: A review. Cancer 1976, 37 (Suppl. 2), 1111–1125.
- Wo, J.Y.; Viswanathan, A.N. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1304–1312.
- Hall, E.J. Cancer caused by x-rays—A random event? Lancet Oncol. 2007, 8, 369–370.
- Maier, P.; Hartmann, L.; Wenz, F.; Herskind, C. Cellular Pathways in Response to Ionizing Radiation and Their Targetability for Tumor Radiosensitization. Int. J. Mol. Sci. 2016, 17, 102.
- Toner, J.P.; Seifer, D.B. Why we may abandon basal follicle-stimulating hormone testing: A sea change in determining ovarian reserve using antimullerian hormone. Fertil. Steril. 2013, 99, 1825–1830.
- Nelson, S.M. Biomarkers of ovarian response: Current and future applications. Fertil. Steril. 2013, 99, 963–969.
- Anderson, R.A.; Wallace, W.H. Antimullerian hormone, the assessment of the ovarian reserve, and the reproductive outcome of the young patient with cancer. Fertil. Steril. 2013, 99, 1469–1475.
- Jung, M.; Shin, H.J.; Rha, S.Y.; Jeung, H.C.; Hong, S.; Moon, Y.W.; Kim, H.S.; Oh, K.J.; Yang, W.I.; Roh, J.K.; et al. The clinical outcome of chemotherapy-induced amenorrhea in premenopausal young patients with breast cancer with long-term follow-up. Ann. Surg. Oncol. 2010, 17, 3259–3268.
- Broekmans, F.J.; Kwee, J.; Hendriks, D.J.; Mol, B.W.; Lambalk, C.B. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum. Reprod. Update 2006, 12, 685–718.
- Knauff, E.A.H.; Eijkemans, M.J.C.; Lambalk, C.B.; Booij, M.J.t.K.; Hoek, A.; Beerendonk, C.C.M.; Laven, J.S.E.; Goverde, A.J.; Broekmans, F.J.M.; Themmen, A.P.N. Anti-Mullerian hormone, inhibin B, and antral follicle count in young women with ovarian failure. J. Clin. Endocrinol. Metab. 2009, 94, 786–792.
- Muttukrishna, S.; McGarrigle, H.; Wakim, R.; Khadum, I.; Ranieri, D.M.; Serhal, P. Antral follicle count, anti-mullerian hormone and inhibin B: Predictors of ovarian response in assisted reproductive technology? BJOG 2005, 112, 1384–1390.
- Frattarelli, J.L.; Levi, A.J.; Miller, B.T.; Segars, J.H. A prospective assessment of the predictive value of basal antral follicles in in vitro fertilization cycles. Fertil. Steril. 2003, 80, 350–355.
- D’Avila, Â.M.; Biolchi, V.; Capp, E.; Corleta, H.V. Age, anti-mullerian hormone, antral follicles count to predict amenorrhea or oligomenorrhea after chemotherapy with cyclophosphamide. J. Ovarian. Res. 2015, 8, 82.
- Anderson, R.A.; Cameron, D.A. Pretreatment serum anti-mullerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J. Clin. Endocrinol. Metab. 2011, 96, 1336–1343.
- Gook, D.A.; Edgar, D.H. Human oocyte cryopreservation. Hum. Reprod. Update 2007, 13, 591–605.
- Practice Committees of the American Society for Reproductive Medicine; The Society for Assisted Reproductive Technology. Intracytoplasmic sperm injection (ICSI) for non-male factor indications: A committee opinion. Fertil. Steril. 2020, 114, 239–245.
- Dittrich, R.; Lotz, L.; Mueller, A.; Hoffmann, I.; Wachter, D.L.; Amann, K.U.; Beckmann, M.W.; Hildebrandt, T. Oncofertility: Combination of ovarian stimulation with subsequent ovarian tissue extraction on the day of oocyte retrieval. Reprod. Biol. Endocrinol. 2013, 11, 19.
- Sánchez, M.; Maestre, E.N.; Teruel, J.; Ortiz, E.; Pellicer, A. The Valencia Programme for Fertility Preservation. Clin. Transl. Oncol. 2008, 10, 433–438.
- Donnez, J.; Dolmans, M.M.; Pellicer, A.; Garcia, C.D.; Serrano, M.S.; Schmidt, K.T.; Ernst, E.; Luyckx, V.; Andersen, C.Y. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: A review of 60 cases of reimplantation. Fertil. Steril. 2013, 99, 1503–1513.
- Suzuki, N. Ovarian tissue cryopreservation in young cancer patients for fertility preservation. Reprod. Med. Biol. 2015, 14, 1–4.
- Dolmans, M.M.; Luyckx, V.; Donnez, J.; Andersen, C.Y.; Greve, T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil. Steril. 2013, 99, 1514–1522.
- Loren, A.W.; Senapati, S. Fertility preservation in patients with hematologic malignancies and recipients of hematopoietic cell transplants. Blood 2019, 134, 746–760.
- Rosendahl, M.; Andersen, M.T.; Ralfkiær, E.; Kjeldsen, L.; Andersen, M.K.; Andersen, C.Y. Evidence of residual disease in cryopreserved ovarian cortex from female patients with leukemia. Fertil. Steril. 2010, 94, 2186–2190.
