Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Cristina Alina Silaghi and Version 2 by Conner Chen.
Intracranial germinomas are rare tumours, usually affecting male paediatric patients. They frequently develop in the pineal and suprasellar regions, causing endocrinological disturbances, visual deficits, and increased intracranial pressure. The diagnosis is established on magnetic resonance imaging (MRI), serum and cerebrospinal fluid (CSF) markers, and tumour stereotactic biopsy. Imaging techniques, such as susceptibility-weighted imaging (SWI), T2* (T2-star) gradient echo (GRE) or arterial spin labelling based perfusion-weighted MRI (ASL-PWI) facilitate the diagnosis.
- CNS germinoma
- KIT
- ß-HCG
- AFP
1. Introduction
Intracranial germ cell tumours (ICGCT) are rare tumours that primarily affect children and adolescents, with a male predominance, accounting for 3.6% of brain tumours in Western Europe and reaching a higher incidence of 15.4% in Japan [1][2][3][1,2,3]. A comparative study between Japanese and American populations regarding ICGCT revealed a different distribution of the tumours. More cases of basal ganglia involvement were present in the Japanese, whereas more bifocal (synchronous pineal and suprasellar) locations in western society, suggesting the presence of genetic or environmental factors, thus, contributing to the phenotypic diversity [4]. According to the WHO classification of the central nervous system (CNS) tumours, the germ cell tumours group, composed of germinoma, represents the most common histological type (60%), followed by mixed germ cell tumours (17.4%), teratoma (15.7%), yolk sac tumour (4.2%), choriocarcinoma (1.6%), and embryonal carcinoma (1.1%) [5]. According to Matsutani et al., pure germinoma and mature teratoma have a good prognosis, but other histological types may result in unfavourable prognosis. Intermediate prognosis corresponds to germinoma with syncytiotrophoblastic giant cell [STGC], immature teratoma, teratoma with malignant transformation and mixed tumours composed mainly of germinoma or teratoma, whereas poor prognosis is found in choriocarcinoma, yolk sac tumour, embryonal carcinoma, as well as in mixed tumours composed mainly of choriocarcinoma, yolk sac tumour, or embryonal carcinoma [6][7][6,7].
Germinomas usually develop in the midline areas of the brain, most often in the pineal gland (50% of the pineal tumours are germinomas) and the suprasellar region [8]. In approximately 5–10% of the cases, the tumour is ectopically situated (in other areas than neurohypophysial or pineal sites), including the basal ganglia, thalamus, corpus callosum, cerebellum, septum pellucidum, temporal lobe, and the spinal cord [9][10][11][12][13][9,10,11,12,13]. Suprasellar germinomas are associated with female patients (<15 years old), while pineal germinomas with male patients (>15 years) [14]. In rare cases, germinomas have been described to occur later in life. Several reports mention the diagnosis in the sixth or seventh decade of life, outlining the fact that germinoma should be included as a differential diagnosis even in the elderly [15][16][17][18][19][15,16,17,18,19]. Germinomas can also be present as synchronous lesions in the pineal region and hypothalamo-neurohypophyseal axis, also referred to as “bifocal germinomas”, with an incidence ranging from to 6% to 26% and even as high as 41% in some studies [20][21][22][23][24][20,21,22,23,24]. Metastatic germinomas caused by the dissemination of tumour cells through the cerebrospinal fluid are reported in approximately 4.5% of the cases, usually after a mean period of 6–7 years after the initial tumour diagnosis [25][26][25,26]. According to the literature, the most common type of metastasis is spinal “drop metastasis” (32.5%), followed by ventricular dissemination (30%), and to a lesser extent, the suprasellar region, corpus callosum, subarachnoid space [26][27][28][26,27,28]. Concomitant metastases to the ventriculoperitoneal shunt can appear in 20% of the cases [26].
2. Clinical Presentation
Tumours of the pineal gland obstruct the posterior wall of the third ventricle and of the aqueduct of Sylvius, resulting in acute hydrocephalus with headaches, nausea, projectile vomiting, papilledema, and lethargy. Generally, a 2 cm pineal tumour can cause obstructive hydrocephalus [29]. When the tumour grows, it has a compressive effect on the nerve pathway connecting the cortex to the oculomotor nuclei and the superior colliculi, resulting in Parinaud’s syndrome: upward gaze palsy, pupillary reflex dysfunction, and convergence-retraction nystagmus [30]. In comparison with other types of pineal tumours, upward gaze palsy seems to be more frequently encountered in pure germinomas (90%), as a consequence to the mesencephalic dysfunction caused by the germinoma’s progression pattern [31]. In cases of pituitary germinoma, visual disturbances can result from optic chiasma compression or perioptic meningeal seeding [32].
Germinomas developed in the suprasellar region or anterior third ventricle may be presented with endocrinopathy at the diagnosis (diabetes insipidus, delayed growth or gonadal function, precocious puberty, menstrual irregularities, visual field/acuity deficits). Neurohypophyseal axis dysfunction may also appear as an adverse effect of the radiotherapy treatment [21][30][33][34][35][21,30,33,34,35]. Diabetes insipidus (DI) is the most common symptom associated with germinomas occurring in the hypothalamic-neurohypophysial region, followed by visual deficits, hypopituitarism, and increased intracranial pressure [36]. Diabetes insipidus seems to be a reliable predictor of tumoral invasion of the hypothalamus and third ventricle even in the absence of MRI evidence of suprasellar and third ventricle disease [37][38][39][37,38,39]. GH (growth hormone) deficiency and hypogonadism are the most frequent endocrine insufficiencies (89–95% of cases), followed by hypothyroidism and hypocortisolaemia, in approximately 50% of patients [40]. Boys with intracerebral germ tumours can develop precocious puberty (isosexual pseudoprecocity) because of beta-human chorionic gonadotropin (β-hCG) secretion of the syncytiotrophoblasts component of the tumour, which stimulates Leydig cells, with subsequent production of testosterone [41][42][41,42].
Patients with basal ganglia/thalamus germinoma often develop hemiparesis, headache, ataxia, cognitive impairment, and mental status alterations, though the severity of clinical picture does not seem to correlate with the tumour size [43][44][43,44]. Cases of optic nerve germinoma, although rare, present with progressive visual deficits, followed by endocrine dysfunction (most often DI), symptoms that may be inconsistent with the MRI findings, causing a delay in diagnosis [45].
A retrospective study on 49 children diagnosed with pure germinomas reported visual impairments as the most common symptom (47.9%), followed by motor dysfunctions (40.8%), frequently including focal or general weakness, hemiparesis, facial palsy. Patients presenting endocrinological symptoms had a significant delay in diagnosis [39]. Approximately one-third of the patients have prolonged symptomatology (>6 months) before the diagnosis, these cases being associated with a higher risk of metastatic disease [46]. A summary of the clinical presentation is shown in Table 1.
Table 1.
Clinical presentation.
| Neurologic Symptoms | Endocrine Symptoms | Ophthalmologic Symptoms |
|---|
| headaches | diabetes insipidus | Parinaud’s syndrome |
| nausea | GH insufficiency | visual field deficits |
| projectile vomiting | hypogonadism | acuity deficits |
| papilledema | secondary hypothyroidism | |
| lethargy hemiparesis |
hypocortisolaemia (secondary adrenal insufficiency) | |
| ataxia | menstrual irregularities | |
| precocious puberty |
Abbreviations: GH: growth hormone.
3. Diagnosis
3.1. Biological Markers
Different biomarkers have been studied in order to establish the optimum diagnosis. A relative correlation between serum and cerebrospinal fluid (CSF) biomarkers and the tumour’s histological category was set. Alpha-fetoprotein (AFP) is elevated in embryonal carcinomas and teratomas, whereas choriocarcinomas and germinomas secrete β-hCG [47][48][47,48]. However, germinomas have an inconsistent secretion of β-hCG [49][50][49,50]. There is no clear cut-off for hCG levels to distinguish germinomas from mixed germ tumours, but it is considered that pure germinomas produce no or mild levels of CSF β-hCG (<50 mUI/mL), in the latter cases being classified as high risk and requiring a more aggressive chemotherapy regimen [37][51][37,51]. A study conducted on 80 germ cell tumours revealed a sensitivity of 78.9% and a specificity of 96.6% for CSF tumour markers (with a cut-off of 50 IU/L for ß-HCG and 25 ng/mL for AFP). Marker positive germinomas, as well as marker negative NGGCT have been reported [52].
Other biomarkers associated with germinomas include elevated lactate dehydrogenase (LDH) and placental alkaline phosphatase (PLAP). These biomarkers, in combination with the radiological finding of a pineal mass, could provide a high suspicion of the histological subtype, especially in cases of heterogeneous tumours, and may represent a way of monitoring the treatment response [53]. Aihara et al. discovered that the CSF PLAP level is a specific marker for pure germinomas, which can provide a reliable diagnosis of intracranial germinoma, in the absence of a histopathological examination [54]. However, Chiba et al. propose that CSF PLAP levels, with a cut-off value of 8 pg/mL, also correlate with the germinoma component in the context of mixed GCT [55]. The diagnostic algorithm of CNS germinomas, including the main serum/CSF biomarkers, is shown in Figure 1.
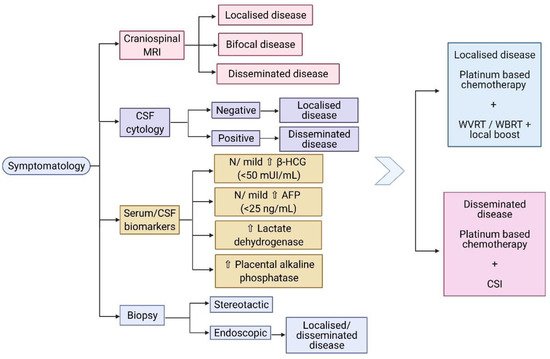
Figure 1. (Created with BioRender.com) Diagnostic algorithm of CNS germinomas. Abbreviations: MRI: magnetic resonance imaging; CSF: cerebrospinal fluid; β-HCG: beta- human chorionic gonadotropin; AFP: alpha fetoprotein; N: normal; WVRT: whole ventricular radiotherapy; WBRT: whole brain radiotherapy; CSI: craniospinal irradiation.
3.2. Radiological Characteristics
On standard MRI, germinomas appear as heterogeneous tumours in T1/T2-weighted imaging, in 40% of the cases, and the uptake of the gadolinium can be either homogeneous (47%) or heterogeneous (53%) [56]. Relevant images of a pure pineal germinoma are shown in Figure 2.
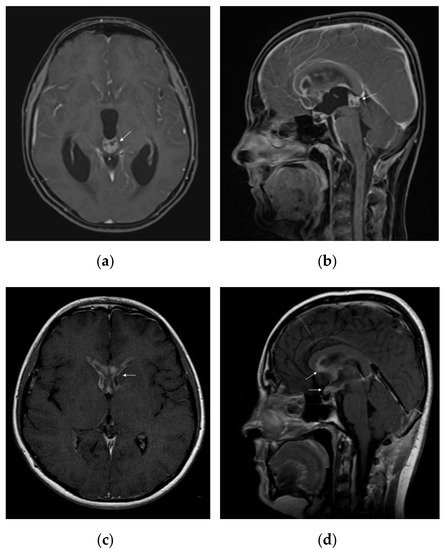
Figure 2. MRI features of intracranial germinoma in a teenage patient: (a) pineal germinoma: heterogeneous contrast enhancement on axial gadolinium-enhanced T1-weighted image, with a tendency to cardioid shape; (b) pineal germinoma: heterogeneous contrast enhancement on sagittal gadolinium-enhanced T1-weighted image; (c) postoperative disseminated disease: bilateral nodular enhancement of anterior horns of the lateral ventricles on axial gadolinium-enhanced T1-weighted image; (d) postoperative disseminated disease: nodular enhancement of the hypothalamus, optic chiasm, mammillary bodies, and corpus callosum on sagittal gadolinium-enhanced T1-weighted image.
Multiple imaging studies have been conducted to provide a better characterization of germinomas. For example, Inoue et al. showed that 90% of the patients with pineal germinomas presented a cardioid-shape tumour image on the axial MRI views, due to its progression pattern on both sides of the third ventricle, concluding that this was a specific aspect for pure pineal germinoma [31]. Awa et al. described two significant features differentiating pineal germinomas from NGGCT: peritumoural edema thicker than 5 mm (peritumoural area with T2 hyperintensity) and bithalamic extension [57]. T2* (T2-star) sequence is generally used to obtain a better characterization of intratumoural/intraventricular/cerebral microhaemorrhage, iron deposits, and calcifications [58]. Susceptibility-weighted imaging (SWI) or T2* gradient echo (GRE) technique can be used for better differentiation between pure germinoma and NGGCT in the pineal region: 93% of the germinomas present iso- or hyperintensity, whereas NGGCT are hypointense compared to the healthy brain [56]. Another imaging technique, such as the arterial spin labelling based perfusion-weighted MRI (ASL-PWI) could be used in differentiating germinomas from NGGCT, based on lower values of relative tumour blood flow encountered in germinomas [59]. Calcification can be present in both germinomatous and non-germinomatous pineal tumours [56].
Suprasellar germinomas seem to develop from the tuber cinereum and median eminence, infiltrating the infundibulum [60]. Therefore, an isolated thickened pituitary stalk may be the first radiological appearance of a hypothalamo-hypophyseal germinoma [61][62][61,62]. However, they have a delay in diagnosis of a median of 1.4 years, due to insidious onset of symptomatology and MRI findings, often suggestive of inflammation (lymphocytic hypophysitis), pituitary adenomas, and secondary neoplasms, with radiological appearance similar to germinomas [63][64][65][63,64,65]. Nonetheless, GCT represent 66.7% of widened pituitary stalk causes in paediatric population, whereas germinomas represent the second etiology (21–31%) of enlarged pituitary stalk in adults [62][66][62,66]. Cases of diabetes insipidus followed by the occurrence of germinoma during the MRI follow-up have been described, highlighting the importance of imaging re-examination or endoscopic biopsy (with higher sensitivity compared to imaging studies) [38][61][38,61]. Usually, pituitary stalk infiltration is reversible following adequate treatment [60].
Basal ganglia and thalamus germinomas may present variable neuroimaging features (cystic lesion, peritumoural oedema, calcification, intratumoural haemorrhage, contrast enhancement, ipsilateral cerebral atrophy) that may impede reaching the correct diagnosis. Nonetheless, ipsilateral hemiatrophy seems to be a characteristic feature of basal ganglia and thalamus germinomas, which may differentiate them from other tumour types [44]. Surprisingly, the number of lesions detected on the MRI does not represent a poor prognosis factor and does not correlate with the overall survival in the setting of an appropriate treatment protocol [67].
3.3. Biopsy
In the management of germinomas, most studies recommend stereotactic biopsy for a definite diagnosis. Balossier et al. have shown that the histopathological diagnosis for pineal biopsies is more accurate with stereotactic procedures than with endoscopic procedures (93.7% vs. 81.1%) [68]. However, several studies emphasized the importance of endoscopic diagnosis, since in patients with pineal germinoma and DI, metastatic lesions to the third ventricular floor are more frequently identified by direct endoscopy than initially diagnosed by MRI [37][38][37,38]. Accordingly, biopsy-diagnosed pineal germinoma in DI patients should be classified as disseminated disease, even in the absence of MRI evidence [38]. Still, a retrospective multicentre study evaluated the necessity of performing biopsy in patients with bifocal tumour, diabetes insipidus, and negative tumour markers. The study included 91 patients with available histologic diagnosis, of which 92% were pure germinomas and germinomas with syncytiotrophoblastic giant cells, concluding that a tumour biopsy is recommended to ensure the proper diagnosis [69]. Another retrospective study revealed that in cases of pituitary germinoma suspected on MRI, the biopsy can reveal another pathology in 22% of the tumours [70]. Moreover, a fairly recent technique that combines endoscopic biopsy with endoscopic ventriculostomy, using a single trajectory, is considered safe and could become an alternative for the dual procedure in pineal germinomas [71].
3.4. Histological Diagnosis
Macroscopically, germinomas are solid, soft, grey-white, homogenous tumours; however, they can rarely present areas of haemorrhage, necrosis, or cystic components. They can be variably encapsulated or poorly circumscribed and infiltrative. Microscopically, they consist of large primordial germ cells (undifferentiated cells), with clear, abundant PAS+ cytoplasm, large, round nuclei, and prominent nucleoli; occasionally syncytiotrophoblastic giant cells may be present [72]. The cells have high mitotic activity and are organized in sheets, lobules, or nests patterns, separated by fibrovascular septae filled with lymphocytic infiltrates. Occasionally, the lymphoplasmacellular reaction is so robust that the granulomatous inflammation can obscure the tumour cells [5]. Therefore, a characteristic histopathological feature of germinoma is the “two-cell pattern”: a massive immune cell population, with a high lymphocytic predominance, dispersed between tumour cells [73].
Immunohistochemistry (IHC) is further used to provide the histological diagnosis. The membrane immunoreactivity for C-kit (transmembrane protein with tyrosine kinase activity), CD30 (tumour necrosis factor receptor), and D2-40 (podoplanin) aid in differentiating germinomas from embryonal carcinoma and yolk sac tumours [5]. The nucleus is usually reactive for OCT 3/4 (octamer binding transcription factor 3/4), SALL4 (sal-like protein 4), UTF1 (undifferentiated embryonic cell transcription factor 1), NANOG (transcription factor in embryonic stem cells), and ESRG (embryonic stem cell-related gene protein), whereas ribosomes are positive for LIN28 (RNA-binding protein LIN28) [74][75][74,75]. Although PLAP is a distinctive marker of primordial cells, its expression is less consistent, being detected in 82% of germinomas. On the other hand, C-kit and OCT 3/4 are more sensible, with 100% staining among germinoma cells. When the germinoma also contains a syncytiotrophoblastic component, these cells are positive for hCG, human placental lactogen (HPL), CD 30, and CK AE1/3 (cytokeratin AE1/3) [72]. A summary of IHC staining and representative histological images from intracranial germinomas are shown in Table 2 and Figure 3 and Figure 4.
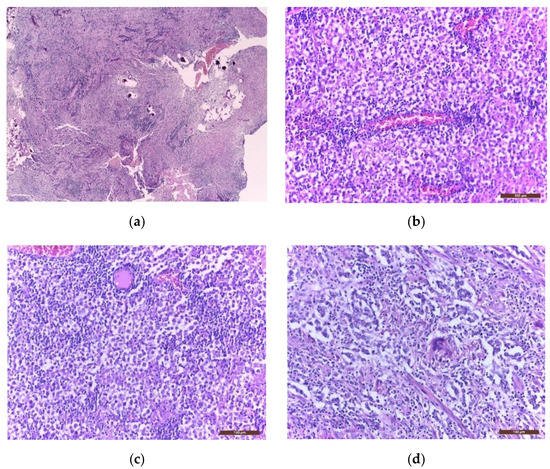
Figure 3. Microscopic images of extragonadal germinomas are identical with those of their gonadal counterpart: (a) pineal gland, with multiple small basophil psammoma bodies, infiltrated by a germinoma; (b) tumour proliferation with fine vascular network; (c) sheets of large germinoma cells, with pale cytoplasm, well defined cell membranes, large round central nuclei, and fibrous septae heavily infiltrated by lymphocytes; (d) an isolated multinucleated syncytiotrophoblast close to the centre of the image; (hematoxylin eosin staining; (a) 40× and (b–d) 200×).
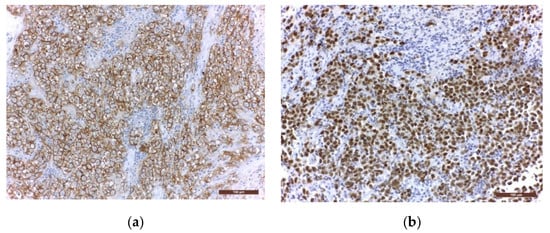
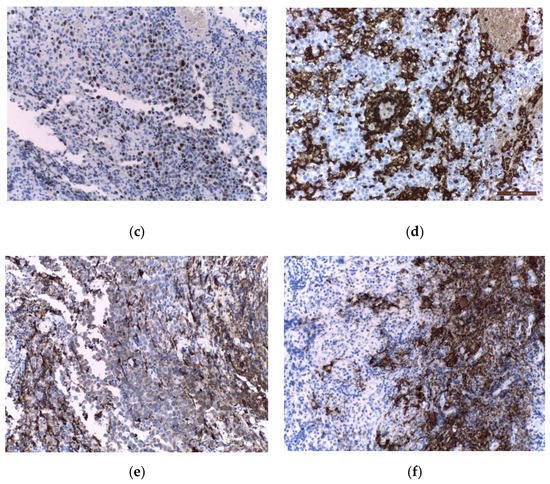
Figure 4. Immunohistochemical staining in extragonadal germinomas: (a) intense membranous and less intense cytoplasmic c-Kit/CD117 expression; (b) intense nuclear and less intense cytoplasmic OCT3/4 expression; (c) high Ki67 nuclear expression; (d) intense expression of leukocyte common antigen LCA/CD45 in stromal lymphocytes; (e) GFAP expression in glial pineal stromal cells in a case of pineal germinoma; (f) synaptophysin expression in the pinealocytes of a pineal germinoma; (200×).
Table 2. Immunohistochemical staining in pure germinomas and germinomas with STGC.
| Staining | Location | Germinoma Cells | Syncytiotrophoblastic Cells |
|---|---|---|---|
| PLAP | Cytoplasm | + | − |
| C-kit | Membrane | + | − |
| OCT 3/4 | Nucleus | + | − |
| HCG | Cytoplasm | − | + |
| AFP | Cytoplasm | − | − |
| CD30 | Membrane | − | + |
| CK AE1/3 | Cytoplasm | − | + |
| D2-40 | Membrane | + | − |
| LIN28 | Ribosomes | + | − |
| HPL | Cytoplasm | − | + |
| NANOG | Nucleus | + | − |
| ESRG | Nucleus | + | − |
| UTF1 | Nucleus | + | − |
| SALL4 | Nucleus | + | − |
Abbreviations: STGC: syncytiotrophoblastic giant cells; PLAP: placental alkaline phosphatase; C-kit: transmembrane protein with tyrosine kinase activity (or CD117); OCT 3/4: octamer binding transcription factor 3/4; HCG: human chorionic gonadotropin; AFP: alpha fetoprotein; CD30: tumour necrosis factor receptor; CK AE1/3: cytokeratin AE1/3; D2-40: podoplanin; LIN28: RNA-binding protein LIN28; HPL: human placental lactogen; NANOG: transcription factor in embryonic stem cells; ESRG: embryonic stem cell-related gene protein; UTF1: undifferentiated embryonic cell transcription factor 1; SALL4: sal-like protein 4.
