The homeostasis of mitochondrial metal ions plays an important role in maintaining mitochondria and cell functions and regulating multiple diseases. In particular, channels and transporters for transporting mitochondrial metal ions are very critical, which can be used as potential targets to treat neurodegeneration, cardiovascular diseases, cancer, diabetes and other metabolic diseases. This review summarizes the current research on several types of mitochondrial metal ion channels/transporters and their functions in cell metabolism and diseases, providing strong evidence and therapeutic strategies for further insights into related diseases.
- mitochondrial metal ion transport
- mitochondrial metal ion homeostasis
- mitochondrial function
- cell metabolism
- disease
1. Introduction
Mitochondria are cytoplasmic organelles crucial to life. Since the major function of mitochondria is to produce a large amount of energy ATP, they are called the powerhouse of the cell. However, the functions of mitochondria are multifaceted, far beyond bioenergetics, such as cell metabolism, apoptosis, fatty acid β -oxidation, reactive oxygen species (ROS) signaling, steroid synthesis, and metal ion homeostasis [1][2][3][4][5][1,2,3,4,5]. Mitochondria have a double membrane structure, namely mitochondrial inner membrane (MIM) and mitochondrial outer membrane (MOM), both of which contain selective and nonselective ion channels and transporters [6][7][6,7]. The MOM with a simple structure is a permeable membrane for small molecules and ions, while the MIM is highly impermeable and its passive transport mode is usually driven by electrochemistry [8][9][8,9]. The recently discovered mitochondrial metal ion channels/transporters are mainly located on these mitochondrial membranes, especially the MIM. While some metal ion channels/transporters participate in normal physiological activities, others only play their roles in pathological states, both of which are essential for cell survival and metabolism. Moreover, mitochondrial metal ion channels/transporters are considered to be important communication media between mitochondria and cytoplasm. Unbalanced communication causes disorders of cell metabolism and energy supply, leading to multiple pathologies [10][11][10,11].
Impaired metal ion homeostasis at the cellular level is linked to mitochondrial dysfunction [12][13][12,13]. The dynamic balance of metal ions inside and outside the mitochondria plays significant roles in numerous cellular physiological processes, including activating ATPase, maintaining ATP production, keeping the homeostasis of mitochondrial volume, regulating the concentration of ROS, controlling signal transduction, and holding the balance of other ion concentrations. Furthermore, it has been reported that the metal ion dyshomeostasis in mitochondria is related to pathological features in neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease [12][14][15][12,14,15]. In addition, diseases related to mitochondrial metal ion dyshomeostasis also include cancer, type 2 diabetes (T2D), heart failure, ischemia and reperfusion injury [16][17][18][19][20][16,17,18,19,20]. It is particularly important to note that targeting certain mitochondrial metal ion channels/transporters can treat diseases, such as hypoxic pulmonary artery hypertension, cancer and neurodegenerative disorders [21][22][23][21,22,23].
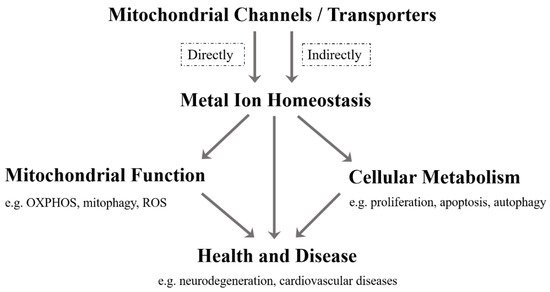
As far as we know, although the current research on mitochondrial metal ion transport is still limited, it has been found that there are several specific channels/transporters on the inner or outer membrane of mitochondria, which are responsible for transporting different metal cations, such as Ca 2 + , K + , Na + , Mg 2+ , Zn 2+ and Fe 2+ /Fe 3+ ( Table 1 ). Mitochondrial channels/transporters and transports of metal cations are the key to modulating metal ion homeostasis directly or indirectly, which is essential for mitochondrial function, cellular metabolism, health and disease ( Figure 1 ). Herein, our review summarizes the current relevant studies and focuses primarily on several types of mitochondrial metal ion transport and their roles in cell metabolism and diseases. Not only can it provide a reference for in-depth research on the transport of mitochondrial metal ions, but is also expected to develop more disease treatment strategies.
| Metal Ions | Mitochondrial Channels/Transporters | Related Diseases | References | |
|---|---|---|---|---|
| Importer/Influx | Exporter/Efflux | |||
| Ca2+ | VDAC, MCU, mRYR | Letm1, NCLX, mPTP | Insulin resistance, T2D, Diabetes-related cardiac disease, Heart failure, Ischemia, Reperfusion injury, Brain aging, Neurodegenerative diseases, Cancer | [17][20][24][25][26][27][28][29][30][31][32][33][34][35][36] |
| K+ | mitoKATP, KCa, Kv, mitoTASK-3 | KHE | Epilepsy, Diabetic cardiomyopathy, Ischemia, Reperfusion injury, Pulmonary artery hypertension, Neurodegeneration, Cancer, Schizophrenia, Sudden cardiac death | [37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53] |
| Na+ | NCLX | NHE | Heart failure, Sudden death, Neurodegenerative diseases | [18][35][54][55][56][57] |
| Mg2+ | MRS2 | SLC41A3, Mme1 | Cancer, Demyelination, Neurodegeneration | [58][59][60][61][62][63][64][65] |
| Zn2+ | MCU, ZnT4 | ZIP8, mitoKATP | Neurodegeneration | [66][67][68][69][70][71] |
| Fe2+/Fe3+ | MFRN, Tf/TfR2, DMT1 | —— | Anemia, Neurodegenerative diseases | [72][73][74][75][76][77][78][79][80][81] |
2. Mitochondrial Iron Ion
Mitochondria are the main utilization sites of iron which are transported to the matrix to synthesize iron-sulfur clusters and heme [24][153]. The precise regulation of iron ions in mitochondria is essential for hemoglobin production, Fe-S cluster protein assembly and heme biosynthesis during red blood cell development [25][26][27][78,154,155]. It is conceivable that mitochondrial iron homeostasis is involved in various hematological diseases. Mitochondrial iron homeostasis and its dysfunctions have been found in sideroblastic anemia and neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, Huntington disease, and Friedreich’s ataxia [25][28][29][30][78,79,80,81]. Strikingly, iron overload is the primary cause of increased morbidity in thalassemia [31][156], but the current research on the relationship between mitochondrial iron homeostasis and thalassemia is very rare. In fact, abnormal cellular iron metabolism is largely affected by mitochondrial iron dyshomeostasis, which may lead to iron overload associated side effects. In turn, an iron loss induced by iron chelator triggers mitophagy [32][157].
MFRN (SLC25A37) belongs to the vertebrate mitochondrial solute carrier protein family, which transports various metabolites and cofactors on the MIM. Some researchers show that the MFRN is a carrier of iron ions into mitochondria, and mitochondria of MFRN mutants disrupt iron ion uptake, leading to severe hypochromic anemia and stagnant red blood cell maturation [33][34][35][36][72,73,74,75]. In mouse embryonic stem cells, the lack of MFRN causes fibroblasts to stop maturing and inhibits heme synthesis [33][72]. The disruption of yeast MFRN orthologs MRS3 and MRS4 leads to defects in iron metabolism and mitochondrial Fe-S cluster formation [37][38][158,159]. The transferrin/transferrin receptor 2 (Tf/TfR2) transport system has been reported to deliver transferrin-bound iron to mitochondria, which is disrupted in Parkinson’s disease [39][76]. Another divalent metal transporter called DMT1 on the MOM also transports iron ions [40][77]. Overexpression of DMT1 is observed to increase the mitochondrial uptake of iron ions driven by proton gradients. Some small solutes and metal ions enter the mitochondrial membrane space through VDAC, which may involve iron uptake. Further research shows that VDAC is one of DMT1 interacting partners, and DMT1–VDAC interactions mediate mitochondrial iron uptake in cells [41][160]. Interestingly, mitochondrial ferritin (FTMT), as a novel iron-storage protein in mitochondria, participates in regulating iron distribution between cytosol and mitochondrial contents and has a protective effect in pathogenesis of neurodegenerative diseases in cell models [42][43][161,162]. It is worth noting that mitochondrial iron ion efflux channels/transporters have not yet been discovered, which requires an in-depth investigation ( Table 1 ).
3. Mitochondrial Manganese Ion
Mitochondria are the main organelles producing ROS, the accumulation of which will cause cell toxicity, accelerated mutagenesis, lipid peroxidation, protein oxidation and cell death. Thus, the primary mechanism to eliminate ROS relies on superoxide dismutase (SOD), including the cytosolic copper/zinc-dependent SOD (Cu/ZnSOD) and the mitochondrial manganese-dependent SOD (MnSOD) [44][163]. MnSOD is the only SOD isoform present in mitochondria, which requires a manganese ion as a cofactor to execute its antioxidant defense function [45][164]. At the cellular level, SLC39A8 (ZIP8) and SLC39A14 (ZIP14) have been identified to specifically mediate manganese uptake in mammals [46][47][48][165,166,167], while SLC30A10 (ZnT10) controls manganese efflux from cells [49][50][168,169]. However, the manganese ion transport mechanism in mitochondria is still unclear. It has been proposed that manganese ion uptake from cytosol to mitochondria is possibly mediated via MCU or MFRN1 [51][170]. Cells lacking MCU are more resistant to Mn 2+ toxicity [52][171]. A potential role of mitochondria-localized SLC39A8 in the regulation of mitochondrial manganese ion transport will be an interesting point in future research. Of note, the potential role for altered Mn homeostasis and toxicity in neurodegenerative disorders has been reported, but whether it is related to mitochondrial manganese ion transport is still unknown [53][54][55][172,173,174].
4. Other Mitochondrial Metal Ions
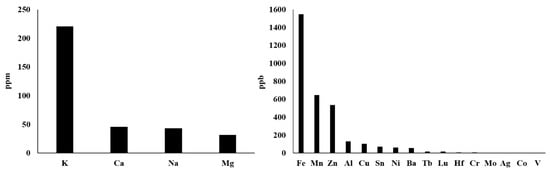
Actually, there are more types of metal ions in mitochondria, not just those described above. With reference to the method of rapid immunopurification of mitochondria [56][57][175,176], we isolated mitochondria from HepG2 cells and measured the content of different mitochondrial metal ions by inductively coupled plasma mass spectrometry (ICP–MS). This ICP–MS method has been reported to provide high accuracy in monitoring various metal ions [58][59][177,178]. As shown in Figure 2 , our results demonstrate that the seven most abundant metal elements in mitochondria are K, Ca, Na, Mg, Fe, Mn and Zn, whose mitochondrial transport channels and transporters have attracted great attention from researchers. So, their transport channels and transporters have been discovered more or less. However, Al, Cu, Sn, Ni, Ba and other metal ions whose abundances are closely followed and relatively low should also be considered seriously. On the one hand, what are the functions of these metal ions in mitochondria? Are they related to some mitochondrial diseases? On the other hand, how they are transported into and out of mitochondria? These questions have not yet been fully answered and require further investigation. For instance, aluminum is considered to be an inducer of the mitochondrial permeability transition [60][179], and aluminum phosphide can induce oxidative stress and mitochondrial damages in cardiomyocytes and isolated mitochondria [61][180]. Unfortunately, the transport of mitochondrial aluminum ions is still unclear. Another example is about mitochondrial copper ions. The study has found mitochondrial copper homeostasis and its derailment in Wilson disease [62][181]. Disruption of mitochondrial copper distribution inhibits self-renewal of leukemia stem cells [63][182]. More interestingly, it has recently been discovered that mitochondrial copper depletion can suppress triple-negative breast cancer [64][183]. These studies indicate that mitochondrial copper may be a potentially important and non-negligible target in therapy. However, mitochondrial copper ion transport is poorly understood. As for the lower contents of Sn, Ni, Ba and other metal ions, it is not yet clear how they are transported between the cytoplasm and the mitochondrial matrix. In brief, it is necessary to comprehensively investigate the transport of these mitochondrial metal ions in cell metabolism and diseases, which may become a potential target for the treatment of related diseases.