Diabetes mellitus (DM) is a prevailing global health metabolic disorder, with an alarming incidence rate and a huge burden on health care providers. DM is characterized by the elevation of blood glucose due either to a defect in insulin synthesis, secretion, binding to receptor, or an increase of insulin resistance. The internal and external factors such as obesity, urbanizations, and genetic mutations could increase the risk of developing DM. Flavonoids are phenolic compounds existing as secondary metabolites in fruits and vegetables as well as fungi. Their structure consists of 15 carbon skeletons and two aromatic rings (A and B) connected by three carbon chains. Flavonoids are furtherly classified into 6 subclasses: flavonols, flavones, flavanones, isoflavones, flavanols, and anthocyanidins. Naturally occurring flavonoids possess anti-diabetic effects. As in vitro and animal model’s studies demonstrate, they have the ability to prevent diabetes and its complications.The aim of this review is to summarize the current knowledge addressing the anti-diabetic effects of dietary flavonoids and their underlying molecular mechanisms on selected pathways: Glucose transporter, hepatic enzymes,tyrosine kinase inhibitor, AMPK, PPAR, and NF-B. Flavonoids improve the pathogenesis of diabetes and its complications through the regulation of glucose metabolism, hepatic enzymes activities, and lipid profile. Most studies illustrate a positive role of specific dietary flavonoids on diabetes, but the mechanisms of action and the side effects need more clarification. Overall, more research is needed to provide a better understanding of the mechanisms of diabetes treatment using flavonoids.
- diabetes mellitus
- flavonoids
- hyperglycemia
- anti-diabetic
- lipogenesis
1. Diabetes and Flavonoids
1.1. Diabetes Mellitus
1.2. Glucose Homeostasis
1.3. Insulin Resistance
1.4. Insulin Release Defect in Diabetes
1.5. Lipogenesis Regulation in Adipocytes
1.6. Diabetes Management
1.7. Impact of Diabetes on Selected Pathways
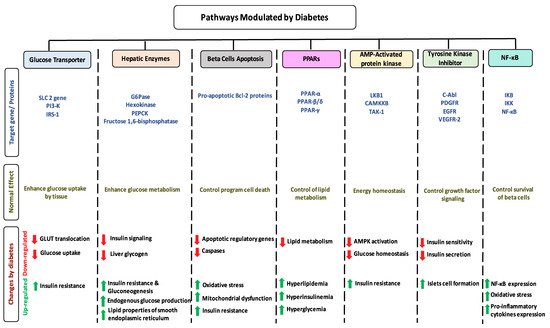
1.8. Dietary Flavonoids
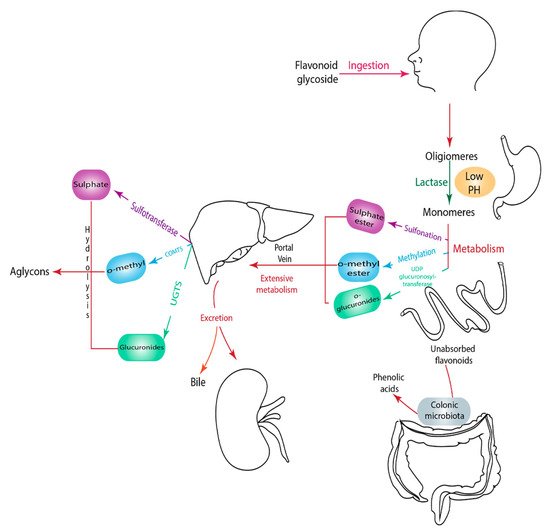
1.9. Metabolism of Flavonoids
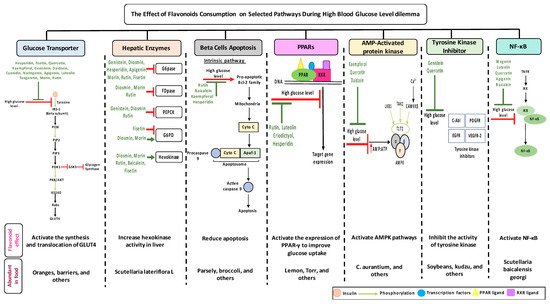
2. Anti-Diabetic Effects of Selected Flavonoids
2.1. Flavonol
2.1.1. Quercetin
| Flavonoid Subclass | Name of Flavonoid | Structure of Flavonoid | Dietary Source | Metabolites Produced from Flavonoids | Function of Flavonoids | Mechanism of Action | Model Used | References | |
|---|---|---|---|---|---|---|---|---|---|
| In Vivo | In Vitro | ||||||||
| Flavonol | 1. Rutin | 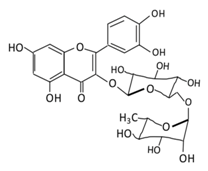 |
Oranges, grapes, limes, lemons, berries and peaches. | Metabolization depends on intestinal bacteria: (A) Bacillus 52 and Bacteroides 45 produce: Quercetin-3-O-glucoside and Leucocynaidin. (B) Bacteroides 42 and veillonella 32 produces: Leucocynaidin. (C) Bacteroides 22 hydrolysis produce: Quercetin-3-O-glucosie |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect |
Inhibit α-glucosidase and α-amylase which reduce the absorption of glucose in small intestine Decrease G6Pase, PEPCK, glycogen phosphorylase, and fructose-1,6-bisphosphatase enzymes in liver and kidney Decrease the level of caspase 3 and increase the level of Bcl-2 which shows an anti-apoptotic activities Reduce the level of hemoglobin A1C (HbA1c) Activate the synthesis and translocation of GLUT4 that stimulate glucose transport to soleus muscle tissue Increase hexokinase activity in liver Improve the morphology of islets of Langerhans Reduce serum LDL, VLDL, triglyceride Inhibit lipid peroxidation Increase serum level of HDL Activate the expression of PPAR-γ which improve glucose uptake and insulin resistance |
Streptozotocin induced diabetic rats Type 2 diabetic rat Streptozotocin induced diabetic wistar rats |
Streptozotocin diabetic tissue |
[81][82][81,83] |
| 2. Fisetin | 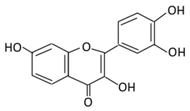 |
Onion, strawberries, and persimmon | (A) Glucuronide of fisetin (M1) (B) Glucuronide (M2) (C) Methoxylated metabolites of fisetin (M3) |
(A) Antihyperglycmeic effect | Inhibit gluconeogenesis by inhibiting pyruvate transport into mitochondria Decrease glycogen breakdown which prevent hyperglycemia Reduce blood glucose, Hb1Ac, IL-1β, and NF-κB p65 unit Reduce the activity of glucose glucose-6-phosphate dehydrogenase activity |
Streptozotocin induced diabetic rats |
[83][84][107,108] | ||
| 3. Kaempferol | 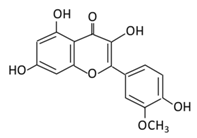 |
Cruciferous vegetables, tea, grapefruit, edible berries, and Gingko biloba L. | (A) Kaempferol-3-O-glucoside (B) Kaempferol-3-O-diglucoside |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect |
Reduce serum glucose level and fasting blood glucose level Decrease the level of caspase 3 activity in β-cells Inhibit cellular apoptosis by improving anti-apoptotic Akt activities Improve cAMP signaling and insulin synthesis and secretion Improve glucose uptake by soleus muscles Reduce lipid peroxidation Decrease PPARγ expression through AMPK activity |
Rats Streptozotocin (STZ)-induced diabetic rats High fat diet mice |
Pancreatic β-cells | [85][86][90,91] | |
| 4. Quercetin | 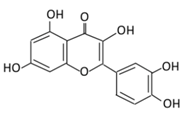 |
Black currants, cherries, apples and chokeberries | (A) Quercetin-3-O-glucoside (B) Quercetin -3-O-glucoside-7-O-glucoside (C) Quercetin-3-O-galactoside (D) Aglycone |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect |
Inhibit insulin dependent activation of PI3K Inhibit GLUT2 which reduces the absorption of glucose in small intestine Block the activity of tyrosine kinase Improve GLUT4 translocation through the activation of AMPK Improve the recovery of cell proliferation Improve glucose absorption Reduce lipid peroxidation |
Rats Streptozotocin (STZ)-induced diabetic rats High fat diet mice |
Skeletal muscle cells Hepatocyte RINm5F β-cells |
[68][69][68,69] | |
| 5. Isorhamnetin | 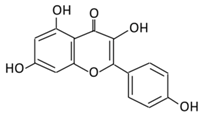 |
Oenanthe javanica, Hippophae rhamnoides, and Ginkgo biloba L. | (A) Isorhamnetin (B) isorhamnetin-3-O-galacto |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect: |
Improve insulin secretion Increase glucose transporter 2 (GLUT2) Inhibit adipogenesis |
HFD- induced C57BL/6 mice | 3 T3-L1 cells | [87][88][102,103] | |
| 6. Morin | 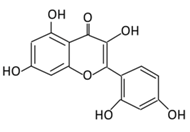 |
Psidium guajava, Prunus dulcis (Almond), chlorophora tinctoria, and fruits | (A) Morin glucuronides (B) Morin sulfates |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect. |
Reduce hepatic NF-κB activation Reduce G6Pase and FDPase enzymatic activities Increase hexokinase and G6PD enzymatic activities Improve hyperglycemia, insulin resistance, and glucose intolerance Reduce lipid peroxidation Reduce hyperlipidemia Normalize the profile of lipid and lipoprotein |
Streptozotocin (STZ)-induced diabetic rats High fructose fed rats HFD-STZ induced type 2 diabetic rats |
Rats hepatocyte | [89][90][114,115] | |
2.1.2. Rutin
2.1.3. Kaempferol
2.1.4. Isorhamnetin
2.1.5. Fisetin
2.1.6. Morin
2.2. Flavanones
2.2.1. Hesperidin
| Flavonoid Subclass | Name of Flavonoid | Structure of Flavonoid | Dietary Source | Metabolites Produced from Flavonoids | Function of Flavonoids | Mechanism of Action | Model Used | References | |
|---|---|---|---|---|---|---|---|---|---|
| In Vivo | In Vitro | ||||||||
| Flavanones | 7.Hesperidin | 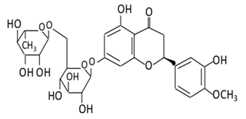 |
Orange citrus aurantium |  |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect |
Down- regulate the production of free radical and proinflammatory cytokines Reduce oxidative stress Reduce blood glucose level by affecting glucose enzymatic activities Increase glycogen concentration and hepatic glycolysis Reduce the level of TBARS which is a byproduct of lipid peroxidation Normalize adiponectin level Increase the activity of lactate dehydrogenase (LDH) |
Alloxan-induceddiabetic rabbits Streptozotocin (STZ)-induced marginal type 1 diabetic rats (10g/kg diet) |
[122][124][122,124] | |
| 8.Naringenin | 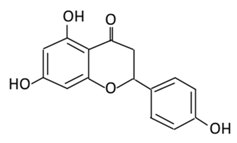 |
Grapefruit (C. paradisi), Chinese herbs like C. aurantium | Four forms could be present in the body two of them are major: (A) Naringenin glucuronides (Major form in serum) (B) Naringenin sulfates ( Major form in liver) (C) Free naringin (Not present in blood stream) D) Free naringenin (Not present in blood stream) |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect |
Reduce poliprotein B secretion in the liver which mimic insulin effect Inhibit intestinal α-glucosidase activity which delays carbohydrates absorption | ||||
2.3.1. Apigenin
2.3.2. Luteolin
2.3.3. Tangeretin
2.3.4. Chrysin
| Flavonoid Subclass | Name of Flavonoid | Structure of Flavonoid | Dietary Source | Metabolites Produced from Flavonoids | Function of Flavonoids | Mechanism of Action | Model Used | References | |
|---|---|---|---|---|---|---|---|---|---|
| In Vivo | In Vitro | ||||||||
| Flavones | 10. Baicalein | 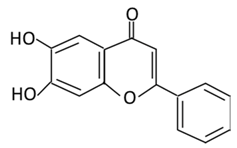 |
Scutellaria lateriflora L, and Scutellaria baicalensis Georgi | In Intestine: Baicalin will be converted into Baicalein and then absorbed rapidly. In the circulation: Baicalein will be converted to Baicalin |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect |
Reduce the level of level of hemoglobin A1C (HbA1c) Suppress the activation of NF-κB Improve glucose tolerance and insulin secretion from pancreatic cells Improve viability of clonal β-cells which improves the production of NADH and NADPH Protect against β cells apoptosis Increase hexokinase activity in liver Activate MAPKs signaling pathway which reduce the effect of insulin resistance by phosphorylating Akt and IRS-1 and dephosphorylate NF-κB Suppress fatty acid synthesis |
Obese diabetic mice Type 2 diabetic rats |
CA1 hippocampal neurons | [172][173] |
| 11. Luteolin | 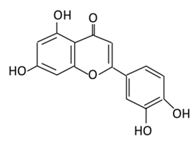 |
Parsley, broccoli, onoins leaves, celery, cabbages, apple skins, carrots, and peppers | Metabolization is medicated by UGTs and COMTs to produce: (A) Luteolin-7-glucuronide (Glucuronidated) (B) Luteolin-4-glucuronide (C) Chrysoeriol/diosmetic (Methylated) (D) Luteolin monoglucuronide (Major form in human serum | Inhibit glucose uptake by inhibiting sodium glucose co-transporter Activate AMPK pathway which increase insulin sensitivity and glucose tolerance Reduce membrane lipid peroxidation Prevent apolipoprotein B overproduction and dyslipidemia Induce hypolipidemic activity |
Streptozotocin (STZ)-induced diabetic rats High fat diet fed mice LDL receptor null mice Male Sprague-Dawley rats |
INS-1E cells | [128][129][133,135] | ||
| 9.Eriodictyol | 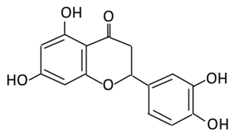 |
Lemon, Torr, Eridictyon californicum, Millettia duchesnei De Wild, and Eupatorium arnottianum | (A) Monoglucuronide M1 in the liver microsome (B) Monoglucuronide M2 in the liver microsome |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect: |
Suppress oxidative stress Decrease Intercellular Adhesion Molecule 1 (ICAM-1), Vascular endothelial growth factor (VEGF), retinal TNFα, and Endothelial NOS (eNOS). Reactivate Akt phosphorylation Reduce lipid peroxidation Up-regulate the expression of PPARγ2 Up-regulate adipocyte- specific fatty acid binding protein |
Streptozotocin induced diabetic rats (0.2%) |
HepG2 cells Differentiated 3T3-L1 cells |
[130][131][144,146] | |
2.2.2. Naringenin
2.2.3. Eriodictyol
2.3. Flavones
| (A) Antihyperglycmeic effect: | ||||||||
| (B) Hypolipemic effect | Reduce cAMP response element binding protein and histone acetyl transferase activity of CBP/p300 (NF-κB coactivator) | Reduce apoptosis Up-regulate the espression of synaptic protein which target brain cells Improve insulin secretion by supressing Maf A through NF-κB signiling pathway Activate PPAR-γ which targets adiponectin, leptin and GLUT4 genes |
Obese mice Streptozotocin induced diabetic rats Diabetic rats |
Endothelium cells Human monocytes cells |
[155][157] | |||
| 12. Diosmin | 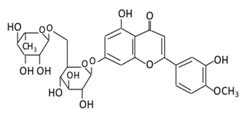 |
Citrus fruites, and Scrophularia nodosa L. | (A) Diosmin (Not excreted in urine) (B) Diosmetin (Not excreted in urine) (C) Minor metabolites in the form of glucuronic acid conjugate (Excreted in urine) |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect: |
Reduce the level of hemoglobin A1C (HbA1c) due to increase in glutathione peroxidase (GPx) Decrease G6Pase, PEPCK, and fructose-1,6-bisphosphatase enzymes Reduce plasma glucose and increase plasma insulin by activating anti-oxidant enzymes Reduce hyperglycemia by inducing β-endorphin Increase hexokinase and glucose-6-phosphate dehydrogenase activity Reduce lipid peroxidation |
Streptozotocin nicotinamide induced diabetic rats |
[174][175] |
2.5. Anthocyanins
2.5.1. Cyanidin
| Flavonoid Subclass | Name of Flavonoid | Structure of Flavonoid | Dietary Source | Metabolites Produced from Flavonoids | Function of Flavonoids | Mechanism of Action | Model used | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In Vivo | In Vitro | ||||||||||||
| Isoflavones | 17. Genistein | 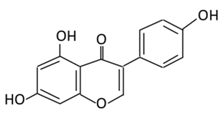 |
Soybeans, kudzu, and fava bean |  |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect |
Reduce hyperglycemia through the activity of cAMP/ PKA pathway Decrease Intercellular Adhesion Molecule 1 (ICAM-1) and p-ERK Inhibit the activity of tyrosine kinase Improve glucose intolerance and β-cells mass Decrease urinary excretion of TBARs |
Streptozotocin (STZ)-induced diabetic rats Obese diabetic mice Nongenetictype 2 diabetic mice |
INS-1 cells Human islet β-cells |
[182][186][195,199] | ||||
| 18. Daidzein | 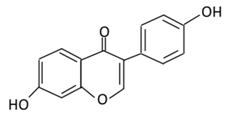 |
Soybeans, nuts, and soy milk | (A) Daidzin | (A) Antihyperglycmeic effect: | Decrease blood glucose, total cholesterol, and AMPK phosphorylation | Golden Syrian hamsters | [189][202[191],204] | ||||||
| Anthocyanins | 19. Cyanidin | 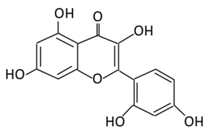 |
Bilberry, blueberry, grapes, blackberries, hawthorn, acai berry, and raspberry | (A) Anthocyanidin glucuronide conjugates (Major form in urine) (B) Simple Aglycones (Second major in urine) (C) Anthocyanidin methyl glucuronide conjugates (8 forms) (D) Cyanidin-3-glucoside E) Cyanidin-3-galactoside |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect: |
Inhibit α-glucosidase and α-amylase which reduce the absorption of glucose in small intestine Reduce fasting glucose level Prevent pancreatic apoptosis Improve antioxidant status which protects hepatocytes from HG-induced damage Attenuate aortic lipid peroxidation |
Streptozotocin (STZ)-induced diabetic rats db/db rats high fat diet fed mice |
Mouse hepatocyte | [194][196][207,209] | ||||
| 13. Apigenin | 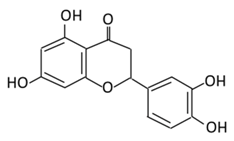 |
Onion, oranges, tea, parsley, chamomile, Hypericum perforatum L, wheat sprouts | Metabolization occurs through two phases: Phase (1): Apigenin produce three monohydroxylated: a) Luteolin b) Scutellarien c) iso-scutellarein Phase(2): Luteolin produce: a) Four monoglucuroconjugates b) Two Sulfoconjugate c) One methyl conjugate |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect: |
|||||||||
| 20.Delphinidin | 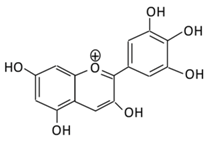 |
Dark grapes, eggplant, berries, red cabbage, carrot, and tomato | (A) 4′-O- methyl delphinidin 3-O-beta-d- glucopyranoside | Reduce cellular antioxidants | Attenuate cell damage in pancreatic β-cells Improve the morphology of the cells Improve GLUT4 translocation which lowers glucose level Increase serum cholesterol Increase lipid peroxidation |
Streptozotocin induced diabetic rats (0.2%) |
HepG2 cells Differentiated3T3-L1 cells |
(A) Antihyperglycmeic effect: | Reduce the glycation rate of HbA1c Prevents diabetes associated injuries such as endothelial cell function | [ | Diabetic mouse147 | ] | [197][149] |
| [ | 198 | ] | [ | 213 | , | 215 | ] | ||||||
| 21.Pelargonidin | 14.Tangeretin | 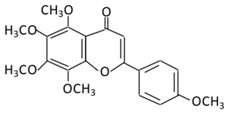 |
Poncirus trifoliate L, citrus fruit rinds, and mandarin orange | Metabolization is medicated by CYP1A1 and CYP1A2 to produce: (A) 4′ hydroxy - 5, 6, 7, 8 tetramethoxyflavone (4′-OH-TMF) |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect: |
Reduce blood glucose and HbA1c level Reduce the secretion of insulin resistance factor Increase the secretion level of insulin and insulin sensitizing factor Enhances glycolytic enzyme in the liver Reduce total cholesterol and adipocytokines level |
Rats Streptozotocin (STZ)-induced diabetic rats High fat diet mice |
Pancreatic β-cells | [160][162] | ||||
| 15. Wogonin | 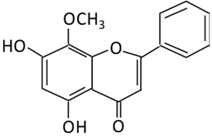 |
Scutellaria baicalensis Georgi | (A) Wogonin-7-beta-D-glucuronide (Major metabolites) (B) Wogonin-5-beta-D-glucuronide |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect: |
Reduce hyperglycemia and lipid droplets accumulation in the liver Increase vascular permeability and the expression of cell adhesion molecules Activate NF-κB and AMPK pathways Activate PPARα which has a beneficial effect on lipid metabolism |
db/db mice | 3T3-L1 cells | [176][177] | |||||
| 16. Chrysin | 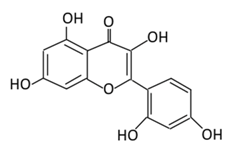 |
passiflora caerulea (L,), honey, Tilia tomentosa Moench, and Pelargonium crispum (Berg.) | (A) Chrysin glucuronides (M1) (B) Chrysin sulfates (M2) |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect: |
Reduce the level of pro-inflammatory cytokines that helps in the prevention of diabetic neuropathy Reduce blood glucose Improve renal pathology with the suppression of TGF-β, collagen-IV, and fibronectin Improve insulin level Reduce lipid peroxidation |
INS-1E cells | [167][169] | ||||||
2.4. Isoflavones
2.4.1. Genistein
2.4.2. Daidzein
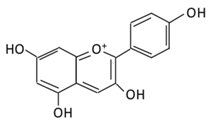 | ||||||||
| Bilberry and | ||||||||
| ficus bengalensis | ||||||||
| Linn | ||||||||
| (A) Pelargonidin- | O | -glucuronide | (B) Pelargonidin-3-galactoside |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect: |
Reduce hyperglycemia Reduce the level of antioxidant defensive enzymes Stimulate insulin secretion Reduce the level of TBARS which is a byproduct of lipid peroxidation |
Streptozotocin (STZ)-induced diabetic rats Diabetic rats |
[199][200][217,219] |
3. Challenges Using Flavonoids
3.1. Estimated Consumption Level of Flavonoids
3.1. Estimated Consumption Level of Flavonoids
Flavonoids derived from vegetables and fruits are consumed in low quantities. Moreover, the content of vegetables and fruits contain not only flavonoids, but also a mixture of secondary plant metabolites. Therefore, it is difficult to stimulate this mixture into a simple purified dietary supplement [201][202][220,221]. Efforts have been made to establish an optimal human dietary consumption level of flavonoids worldwide, but the estimate methods used were poorly established [203][222]. A U.S. study on 805 men aged 65–84 years reported that the estimate intake of flavonoids from quercetin, myricetin, kaempferol, apigenin, and leuteolin was 26 mg/d and the major sources of intake were in apples, tea, and onions [204][223]. Another study conducted in the Netherlands reported a two-times higher the level of flavonoids consumed in adults compared to the U.S. study (50 mg/day) [205][224]. In addition, two Dutch studies reported the estimated consumption level of flavonoids to be 23 mg/day and 26 mg/day respectively [206][207][225,226]. These differences observed in the consumption level of flavonoids depend on dietary habits, geographical location, socioeconomic status, food processing and preparation method, solubility of flavonoids, and the ethnicity of the population. For example, in Japan, soy containing food is highly consumed and, as a result the intake of isoflavone is higher than other flavonoids subclasses [110][106]. A study reported that orange juice contains 81–200mg/L of soluble flavanones compared to 206–644 mg/L seen in the cloud which clearly suggest that processing and storage affects the concentration of flavonoids [208][227].
3.1.1. Possible Side Eects of Flavonoids Consumption
Flavonoids in bacterial and mammalian experimental studies using Ames test indicated possible genotoxicity and mutagenicity of flavonoids if consumed at higher concentrations (ranges from 12.1 nmol to 225.0 nmol) [209]. Furthermore, it may alter amino acid, drug metabolism and the activity of key metabolizing enzymes [210]. Quercetin, a predominant flavonol in the human diet, showed a mutagenic effect by altering base-pair substitution and frame-shift mutation [211]. The isolated nuclei from liver rats treated with morin and naringenin showed an increase in reactive oxygen species, like hydroxyl radicals that lead to DNA degradation [212]. Additionally, flavonoids exert a cytotoxic activity as a topoisomerase II inhibitor. Genistein and quercetin are identified as topoisomerase II inhibitors, even at low concentrations (10 μM), where they accumulate cleavable complexes seen in patients with secondary leukemia [213]. Genistein, naringenin, kaempferol, and daidzein were reported to inhibit thyroxine synthesis by irreversibly inhibiting thyroid peroxidation [214]. Although no data are available to state the long-term side effects of increased flavonoid consumption, following an Asian diet that contains 68 mg of flavonol and 20–240 mg of isoflavone could improve thyroid function, reduce breast cancer mortality, and should not cause adverse health effects [215]. The concentrations needed for most flavonoids to generate mutagenic and cytotoxic side effects are unlikely to occur through dietary sources, but with supplementation, it could result in an increased toxic level. For instance, the recommended dosage of quercetin supplements is between 500 mg/day and 1000 mg/day, which is 20 times higher with what could be consumed in a vegetarian diet [216].Flavonoids in bacterial and mammalian experimental studies using Ames test indicated possible genotoxicity and mutagenicity of flavonoids if consumed at higher concentrations (ranges from12.1 nmol to 225.0 nmol) [228]. Furthermore, it may alter amino acid, drug metabolism and the activity of key metabolizing enzymes [229]. Quercetin, a predominant flavonol in the human diet, showed a mutagenic eect by altering base-pair substitution and frame-shift mutation [230]. The isolated nuclei from liver rats treated with morin and naringenin showed an increase in reactive oxygen species, like hydroxyl radicals that lead to DNA degradation [231]. Additionally, flavonoids exert a cytotoxic activity as a topoisomerase II inhibitor. Genistein and quercetin are identified as topoisomerase II inhibitors, even at low concentrations (10 M), where they accumulate cleavable complexes seen in patients with secondary leukemia [232]. Genistein, naringenin, kaempferol, and daidzein were reported to inhibit thyroxine synthesis by irreversibly inhibiting thyroid peroxidation [233]. Although no data are available to state the long-termside effects of increased flavonoid consumption, following an Asian diet that contains 68 mg of flavonol and 20–240 mg of isoflavone could improve thyroid function, reduce breast cancer mortality, and should not cause adverse health effects [234].The concentrations needed for most flavonoids to generate mutagenic and cytotoxic side effects are unlikely to occur through dietary sources, but with supplementation, it could result in an increased toxic level. For instance, the recommended dosage of quercetin supplements is between 500 mg/day and 1000 mg/day, which is 20 times higher with what could be consumed in a vegetarian diet [235].
3.1.2. Could Flavonoid Combinations have synergistic effects?
While the amounts of flavonoids consumed is crucial to establish positive effects but also to avoid negative effects, the tables list some flavonoids that trigger multiple selected pathways improving the pathogenesis of diabetes (Figure 3, Table 1, Table 2, Table 3 and Table 4). The better activity can be defined by the number of diabetes related pathways which are improved through the consumption of different flavonoids. The administration of baicalein triggers four pathways: The suppression in the NF-κB pathway and fatty acid synthesis; the activation in hexokinase activity in the liver; and the protection against cell apoptosis. Quercetin prompts the activity of three different pathways: It improves GLUT 4 translocation; inhibits tyrosine kinase activity; and reduces lipid peroxidation. β-cells apoptosis could be prevented by the administration of cyanidin or kaempferol, or baicalein. The consumption of rutin or cyanidin inhibits α-glucosidase and α-amylase which reduce carbohydrate absorption in the small intestine (Table 4).While the amounts of flavonoids consumed is crucial to establish positive eects but also to avoid negative effects, the tables list some flavonoids that trigger multiple selected pathways improving the pathogenesis of diabetes (Figure 3, Tables 1–4). The better activity can be defined by the number of diabetes related pathways which are improved through the consumption of different flavonoids.The administration of baicalein triggers four pathways: The suppression in the NF-B pathway and fatty acid synthesis; the activation in hexokinase activity in the liver; and the protection against cell apoptosis. Quercetin prompts the activity of three dierent pathways: It improves GLUT 4 translocation; inhibits tyrosine kinase activity; and reduces lipid peroxidation. -cells apoptosis could be prevented by the administration of cyanidin or kaempferol, or baicalein. The consumption of rutin or cyanidin inhibits -glucosidase and -amylase which reduce carbohydrate absorption in the small intestine (Table 4).
Could their positive effects on diabetes be further improved by ingesting a combination of different flavonoids which complement each other by triggering additional pathways? For example, the administration of baicalein and quercetin initiates the positive effects on diabetes in six major pathways: The glucose transporter; hepatic enzymes; beta cells apoptosis; PPARs; AMPK; tyrosine kinase; and NF-κB pathways. As a result of this hypothesized combination, the over activation of these pathways may be prevented, while the needed action to improve diabetes may be achieved. At this time, these are no more than suggestions which need to be proven by research. To date, little is known about flavonoids to flavonoids interactions [216]. In addition, some flavonoids showed an opposite effect on the same pathway and both lead to the improvement of diabetes. For example, fisetin has an inhibitory effect, while morin has a stimulatory effect on glucose 6 phosphate dehydrogenase and the literature states that they both improve diabetes (Figure 1). Extensive studies are required to understand the reasons behind this action—is it because of different binding sites, bioavailability, tissue exposure, absorption, or circulating concentration of these compounds. A similar pattern with different flavonoids was observed with PPAR and NF-κB pathways (Table 1, Table 2, Table 3 and Table 4).Could their positive effects on diabetes be further improved by ingesting a combination of different flavonoids which complement each other by triggering additional pathways? For example, the administration of baicalein and quercetin initiates the positive eects on diabetes in six major pathways: The glucose transporter; hepatic enzymes; beta cells apoptosis; PPARs; AMPK; tyrosine kinase; and NF-B pathways. As a result of this hypothesized combination, the over activation of these pathways may be prevented, while the needed action to improve diabetes may be achieved. At this time, these are no more than suggestions which need to be proven by research. To date, little is known about flavonoids to flavonoids interactions [235]. In addition, some flavonoids showed an opposite effects on the same pathway and both lead to the improvement of diabetes. For example, fisetin has an inhibitory effects, while morin has a stimulatory effects on glucose 6 phosphate dehydrogenase and the literature states that they both improve diabetes (Figure 1). Extensive studies are required to understand the reasons behind this action—is it because of different binding sites, bioavailability, tissue exposure, absorption, or circulating concentration of these compounds. A similar pattern with different flavonoids was observed with PPAR and NF-B pathways (Tables 1–4).
