Metastasis is the process whereby cancer cells migrate from the primary tumour site to colonise the surrounding or distant tissue or organ. Metastasis is the primary cause of cancer-related mortality and approximately half of all cancer patients present at diagnosis with some form of metastasis. MicroRNAs (miRNAs), a class of small (19-25 nucleotides) non-coding single-strand RNAs, regulate gene expression and play an important role in cancer development and progression including in the metastatic process.
- tumour microenvironment
- miRNAs
- metastasis
1. Introduction
Cancer metastasis, the spread of tumour cells from the primary tumour site, has been reported to account for approximately 67–90% of cancer-related deaths. Approximately half of all cancer patients present with metastasis at the time of diagnosis [1,2,3][1][2][3]. Metastasis is a multistep process, which starts when tumour cells detach from the primary tumour mass, intravasate into lymphatic and circulatory systems to become circulating tumour cells (CTCs), extravasate to leave the circulation, invade and proliferate in a new niche of a distant tissue/organ to form a new tumour [4]. The metastatic process is very inefficient since from the 0.2% of CTCs that survive their time in circulation, only those cells that are the first to reach permissive target organs and are then able to colonize those tissues can initiate metastatic tumour growth [5].
It is well-known that cancer initiation and progression, as well as metastasis, not only depends on tumour cells themselves, but also on the cells of the tumour microenvironment (TME) [6,7,8][6][7][8]. The major components of the TME apart from tumour cells include cancer-associated fibroblasts (CAFs), endothelial cells and immune cells, in addition to other components such as the extracellular matrix [9,10,11][9][10][11]. Hypoxia, cellular oxygen deprivation, is an important factor that drives many aspects of metastasis [12[12][13],13], including the initiation of the epithelial–mesenchymal transition (EMT) process that changes the phenotype of tumour cells allowing them to escape from the matrix of the primary tumour [14]. In addition to hypoxia, the interaction between tumour cells and the TME induces a wide range of biological events that are necessary for metastasis including proliferation, immunosuppression and angiogenesis [15,16,17][15][16][17]. Many of these processes are regulated by microRNAs (miRNAs) and are the subject of this review. In addition to the direct control of metastasis by miRNAs, it has been recently shown that they can regulate the metastatic process by acting as mediators of intercellular communication between tumour cells and cells of the TME [18,19][18][19].
MiRNAs are a class of small (19–25 nucleotides) non-coding single-strand RNAs. Since their initial discovery in Caenorhabditis elegans [20], miRNAs have been demonstrated to play key roles in many, if not all, physiological cellular functions by regulating target genes through primarily negative post-transcriptional regulation of gene expression [21,22,23][21][22][23]. A single miRNA is capable of targeting many genes and, conversely, a single gene can be targeted by many miRNAs leading to a complex regulatory network that encompasses more than 60% of human genes [24]. In addition to their importance under physiological conditions, miRNAs are ubiquitously deregulated in cancer and can act as tumour-promoting miRNAs (oncomiRNAs and metastamiRNAs) targeting messenger RNAs (mRNAs) coding for proteins that act as tumour suppressors or as tumour suppressor miRNAs targeting mRNAs coding for proteins with oncogenic properties [25].
In 2007, two separate reports released in parallel first described the association between miRNAs and metastasis. Ma et al. demonstrated that miR-10b could promote breast cancer metastasis in vitro and in vivo through targeting of the HOXD10 (Homeobox D10) gene [26], whilst Yu et al. demonstrated that let-7 can act as a metastasis suppressor miRNA through targeting of H-RAS and HMGA2 (High Mobility Group AT-Hook 2), leading to a reduction in proliferation, mammosphere formation and metastatic potential, including in breast cancer [27]. Subsequently, many miRNAs have been identified that are associated with metastasis or with associated pathways, such as migration and invasion [28].
2. Biogenesis and Delivery of miRNAs
MiRNA biogenesis starts with the transcription of pri-miRNA sequences from the DNA, with approximately half of miRNAs encoded within intragenic sequences, mainly from introns with the remainder transcribed from intergenic regions and regulated by specific promoter regions [29]. Approximately half of pri-miRNAs encode for multiple miRNAs in a cluster. MiRNA biogenesis can follow either canonical or non-canonical pathways [30]. The canonical miRNA biosynthetic pathway starts with transcription of the pri-miRNA sequence by RNA polymerase II/III; then, RNase-III endonucleases Drosha in concert with the DGCR8 (DiGeorge syndrome critical region 8) cofactor cleave pri-miRNA to form a hairpin pre-miRNA structure [31]. DGCR8 acts to recognize motifs within the pri-miRNA, such as N6-methyladenylated GGAC, while Drosha cleaves pri-miRNA at the base of the structure [31,32][31][32]. The resultant pri-miRNA hairpin structure is exported to the cytoplasm by the exportin-5 (XPO5)/RanGTP complex [33], where it is processed by the RNAse III endonuclease Dicer, which removes the terminal loop of the pre-miRNA structure, resulting in a mature miRNA duplex (Figure 1) [34]. The duplex separates into single-strand effector miRNAs which are loaded into the Argonaute (AGO) protein to form the RNA-induced silencing complex (RISC) that regulates expression of target genes through binding of the miRNA to (primarily) the 3′UTR region of mRNA [35[35][36],36], although instances exist whereby miRNAs can bind to the 5’UTR region, promoter regions and even the coding sequence [37,38,39][37][38][39]. Gene regulation by miRNAs primarily occurs at the post-transcriptional stage and many mechanisms have been described, the majority of which act negatively although positive regulation has also been described [40].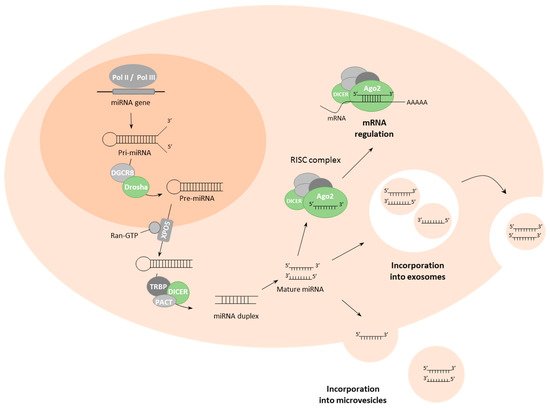
| miRNA | Cancer | Donor Cells | Receptor Cells | Target | Ref. |
|---|---|---|---|---|---|
| miR-9 | Breast | Tumour cells | Fibroblasts | E-cadherin | [71][51] |
| miR-10b-5p | HCC | Tumour cells | Tumour cells | - | [52] |
| miR-15b-5p | GBM | Tumour cells | Non-tumour brain cells | MAPK/ERK | [51][53] |
| miR-17-5p | CRC | CAFs | Tumour cells | RUNX3 | [74][54] |
| miR-19b-3p | ccRCC | CSCs | Tumour cells | PTEN | [59][55] |
| miR-20a-5p | Breast | Tumour cells | BMMs | SRCIN1 | [62][56] |
| miR-21 | Lung | Tumour cells | Pre-osteoclasts | PTEN | [65][57] |
| Lung | Tumour cells | Macrophages | TLR8 | [90][58] | |
| miR-21-5p | GBM | Tumour cells | Non-tumour brain cells | MAPK/ERK | [51][53] |
| HCC | Tumour cells | Tumour cells | - | [52] | |
| Breast | Tumour cells | Osteoclasts | PDCD4 | [64][59] | |
| Breast | CAFs | Tumour cells | - | [73][60] | |
| Bladder | Tumour cells | Macrophages | PTEN | [91][61] | |
| Colon | TAMs | Tumour cells | BRG1 | [98][62] | |
| miR-25-3p | CRC | Tumour cells | Endothelial cells | KLF2/KLF4 | [82][63] |
| miR-29a | Lung | Tumour cells | Macrophages | TLR8 | [90][58] |
| miR-30c-5p | GBM | Tumour cells | Non-tumour brain cells | MAPK/ERK | [51][53] |
| miR-30d-5p | GBM | Tumour cells | Non-tumour brain cells | MAPK/ERK | [51][53] |
| miR-103a-3p | HCC | Tumour cells | Endothelial cells | VE-Cadherin | [81][64] |
| miR-105 | Breast | Tumour cells | Endothelial cells | ZO-1 | [83][65] |
| miR-122 | Breast | Tumour cells | Fibroblasts/astrocytes | PKM2/GLUT1 | [67][66] |
| miR-130b-3p | Gastric | TAMs | Tumour cells | MLL3/GRHL2 | [99][67] |
| miR-143-3p | Breast | CAFs | Tumour cells | - | [73][60] |
| miR-155-5p | Colon | TAMs | Tumour cells | BRG1 | [98][62] |
| miR-210 | Breast | Tumour cells | Endothelial cells | Ephrin A3 | [84][68] |
| Breast | Tumour cells | Endothelial cells | Ephrin A3/PTP1B | [85][69] | |
| miR-210-3p | HCC | Tumour cells | Endothelial cells | SMAD4/STAT6 | [86][70] |
| miR-211 | Melanoma | Tumour cells | Fibroblasts | IGF2R | [72][71] |
| miR-214 | Lung | Tumour cells | Treg | PTEN | [94][72] |
| miR-218-5p | Breast | Tumour cells | Pre-osteoblasts | Col1a1 | [63][73] |
| miR-223-3p | Breast | TAMs | Tumour cells | Mef2c | [101][74] |
| miR-301a-3p | Pancreatic | Tumour cells | Macrophages | PTEN | [93][75] |
| miR-342-3p | OSCC | High-metastatic cells | Low-metastatic cells | - | [60][76] |
| miR-378e | Breast | CAFs | Tumour cells | - | [73][60] |
| miR-501-3p | PDAC | TAMs | Tumour cells | TGDBR3 | [100][77] |
| miR-934 | CRC | Tumour cells | Macrophages | PTEN | [92][78] |
| miR-939-5p | Breast | Tumour cells | Endothelial cells | VE-cadherin | [80][79] |
| miR-940 | Prostate | Tumour cells | MSCs | ARHGAP1 FAM134A |
[66][80] |
| miR-1246 | OSCC | High-metastatic cells | Low-metastatic cells | DENND2D | [60][76] |
| miR-1247-3p | HCC | High-metastatic cells | Fibroblasts | B4GALT3 | [70][81] |
3. MiRNAs in Intercellular Communication in the TME
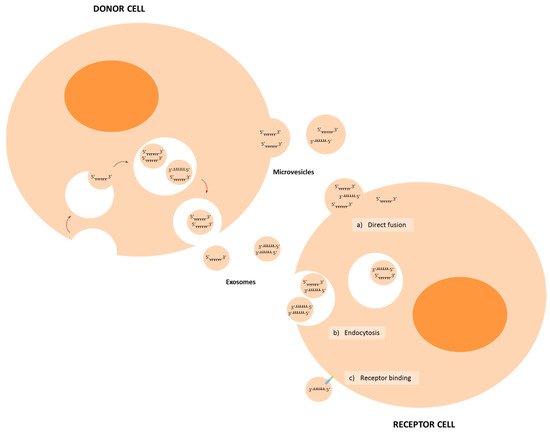
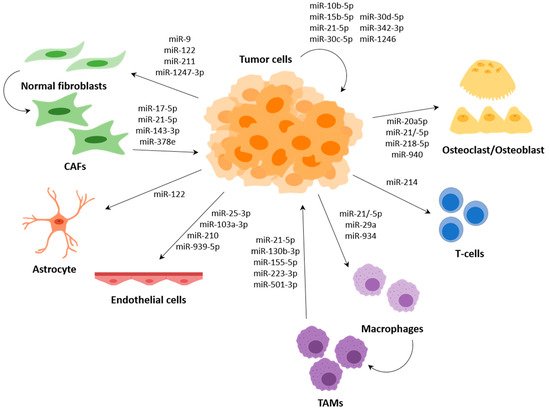
Several miRNAs have been described to participate in the communication between tumour cells and cells of the TME, such as fibroblasts, endothelial cells or immune cells, among others; and several of them regulate expression of genes that are involved in the metastasis process (Figure 3).
3.1. Surrounding Tumour Cells and Premetastasis Niche Formation
3.2. Cancer-Associated Fibroblasts (CAFs)
3.3. Endothelial Cells
3.4. Immune System Modulation by miRNAs
Macrophages are the most abundant infiltrative immune cells present in and around tumours and play a critical role in inflammation [87][96]. Macrophages are known to polarize, depending on different stimuli, to the M1 phenotype with anti-tumour activity or to the M2 phenotype with pro-tumoral activity. Tumour-associated macrophages (TAMs), which are considered to be M2-like, support different aspects of tumour development, including tumour formation, growth and metastasis [88,89][97][98].
The premetastatic inflammatory response generated by TAMs leads to tumour growth and metastasis. MiR-21 and miR-29 secreted by lung tumour cells target TLR8 (toll-like receptor 8) within intracellular endosomes leading to induction of NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) and NF-κB-mediated secretion of the proinflammatory cytokines TNF-α (tumour necrosis factor alpha) and IL-6 (interleukin-6) [90][58]. In bladder cancer, macrophages take up exosomal miR-21-5p from tumour cells leading to promotion of M2 polarization and enhanced migration and invasion of tumour cells [91][61]. Both CRC cell-derived exosomal miR-934 and hypoxic pancreatic cell-derived exosomal miR-301a-3p were demonstrated to activate PI3K/AKT (phosphatidylinositol 3-kinase/protein kinase B) signalling pathway and enhance metastatic capacity of tumour cells through PTEN targeting [92,93][78][75]. PTEN also plays an important role in the regulation of T cells, and it has been demonstrated that EV-associated miR-214 from a range of tumour cells including breast cancer, hepatocellular carcinoma, non-small-cell lung cancer (NSCLC) or pancreatic cancer could transfer to T cells leading to downregulation of PTEN and promoting T-reg (regulatory T cells) expansion and IL-10 (interleukin-10) secretion, which in turn promotes tumour growth and enhanced immune suppression in vivo [94][72].
MicroRNAs have also been shown to be involved in the recruitment of immune cells to the TME, but rather than through the transfer of miRNAs from tumour cells to immune cells, this occurs through the secretion of attractant molecules. For example, both miR-149 in triple-negative breast cancer (TNBC) and miR-148b in HCC have been demonstrated to target colony-stimulating factor-1 (CSF-1) miRNAs [95,96][99][100]. In TNBC, downregulation of miR-149 promoted lung metastasis by enhancing CSF1-dependent recruitment and M2 polarization of macrophages, which also correlated with macrophage infiltration and reduced survival in patient samples [95][99]. Downregulation of miR-148b in HCC patients correlated positively with recurrence, metastasis and poor prognosis. Moreover, in vitro and in vivo metastatic HCC cells showed decreased levels of miR-148b that correlated with increased CSF1, which promoted HCC growth and metastasis through CSF1/CSF1R (colony-stimulating factor-1 receptor)-mediated TAM infiltration [96][100]. Similarly, miR-561-5p, which directly target chemokine (C–X3–C motif) ligand 1 (CX3CL1), in metastatic HCC downregulated CX3CL1 leading to low infiltration of CX3CR1 (CX3C chemokine receptor 1)-positive NK cells and resulting in promoted tumorigenesis and metastasis [97][101].
Moreover, it has been described that TAMs in the TME can release extracellular vesicles with miRNAs, and these can be transferred to tumour cells, generally promoting migration, invasion and metastasis. In CRC, exosomal miR-21-5p and miR-155-5p from macrophages directly target the BRG1 coding gene in tumour cells; this gene is a key factor promoting metastasis [98][62]. Another example is macrophage-derived EVs in gastric cancer (GC), which contain high levels of miR-130b-3p and promote survival, migration, invasion and angiogenesis in GC cells through the modulation of MLL3 (mixed-lineage leukemia protein 3) and GRHL2 (grainyhead-like protein 2 homolog) [99][67]. MiR-501-3p has been found to be highly expressed in pancreatic ductal adenocarcinoma (PDAC) tissues and TAM-derived exosomes. Exosomal miR-501-3p promotes cancer cell migration and invasion, as well as tumour formation and metastasis in vivo through regulation of TGFBR3 (transforming growth factor beta receptor 3) [100][77]. Finally, a study detected miR-223-3p in exosomes released by IL-4 (interleukin-4)-activated macrophages; this miRNA has been shown to transfer to breast tumour cells where it regulates invasion through the Mef2c (myocyte enhancer factor 2C)-β-catenin pathway [101][74].
References
- Robert, J. Biology of cancer metastasis. Bull. Du Cancer 2013, 100, 333–342.
- Sole, C.; Arnaiz, E.; Manterola, L.; Otaegui, D.; Lawrie, C.H. The circulating transcriptome as a source of cancer liquid biopsy biomarkers. Semin. Cancer Biol. 2019, 58, 100–108.
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70.
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73.
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572.
- Naito, Y.; Yoshioka, Y.; Yamamoto, Y.; Ochiya, T. How cancer cells dictate their microenvironment: Present roles of extracellular vesicles. Cell. Mol. Life Sci. Cmls 2017, 74, 697–713.
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458.
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437.
- Giraldo, N.A.; Sanchez-Salas, R.; Peske, J.D.; Vano, Y.; Becht, E.; Petitprez, F.; Validire, P.; Ingels, A.; Cathelineau, X.; Fridman, W.H.; et al. The clinical role of the TME in solid cancer. Br. J. Cancer 2019, 120, 45–53.
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322.
- Rossi, G.R.; Trindade, E.S.; Souza-Fonseca-Guimaraes, F. Tumor Microenvironment-Associated Extracellular Matrix Components Regulate NK Cell Function. Front. Immunol. 2020, 11, 73.
- Ackerman, D.; Simon, M.C. Hypoxia, lipids, and cancer: Surviving the harsh tumor microenvironment. Trends Cell Biol. 2014, 24, 472–478.
- Huang, Y.; Lin, D.; Taniguchi, C.M. Hypoxia inducible factor (HIF) in the tumor microenvironment: Friend or foe? Sci. China Life Sci. 2017, 60, 1114–1124.
- Lu, X.; Kang, Y. Hypoxia and hypoxia-inducible factors: Master regulators of metastasis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 5928–5935.
- Moriwaki, K.; Asahi, M. Augmented TME O-GlcNAcylation Promotes Tumor Proliferation through the Inhibition of p38 MAPK. Mol. Cancer Res. MCR 2017, 15, 1287–1298.
- Lei, X.; Lei, Y.; Li, J.K.; Du, W.X.; Li, R.G.; Yang, J.; Li, J.; Li, F.; Tan, H.B. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020, 470, 126–133.
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Reviews. Cancer 2017, 17, 457–474.
- Soon, P.; Kiaris, H. MicroRNAs in the tumour microenvironment: Big role for small players. Endocr. Relat. Cancer 2013, 20, R257–R267.
- Chou, J.; Shahi, P.; Werb, Z. microRNA-mediated regulation of the tumor microenvironment. Cell Cycle (Georget. Tex.) 2013, 12, 3262–3271.
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854.
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297.
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233.
- Berindan-Neagoe, I.; Monroig Pdel, C.; Pasculli, B.; Calin, G.A. MicroRNAome genome: A treasure for cancer diagnosis and therapy. CA A Cancer J. Clin. 2014, 64, 311–336.
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105.
- Gambari, R.; Brognara, E.; Spandidos, D.A.; Fabbri, E. Targeting oncomiRNAs and mimicking tumor suppressor miRNAs: Νew trends in the development of miRNA therapeutic strategies in oncology (Review). Int. J. Oncol. 2016, 49, 5–32.
- Ma, L.; Teruya-Feldstein, J.; Weinberg, R.A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 2007, 449, 682–688.
- Yu, F.; Yao, H.; Zhu, P.; Zhang, X.; Pan, Q.; Gong, C.; Huang, Y.; Hu, X.; Su, F.; Lieberman, J.; et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell 2007, 131, 1109–1123.
- Solé, C.; Lawrie, C.H. MicroRNAs and Metastasis. Cancers 2019, 12, 96.
- de Rie, D.; Abugessaisa, I. An integrated expression atlas of miRNAs and their promoters in human and mouse. Nat. Biotechnol. 2017, 35, 872–878.
- Forrest, A.R.R.; de Hoon, M.J.L.; Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Biotechnol. 2014, 15, 509–524.
- Denli, A.M.; Tops, B.B.; Plasterk, R.H.; Ketting, R.F.; Hannon, G.J. Processing of primary microRNAs by the Microprocessor complex. Nature 2004, 432, 231–235.
- Alarcón, C.R.; Lee, H.; Goodarzi, H.; Halberg, N.; Tavazoie, S.F. N6-methyladenosine marks primary microRNAs for processing. Nature 2015, 519, 482–485.
- Okada, C.; Yamashita, E.; Lee, S.J.; Shibata, S.; Katahira, J.; Nakagawa, A.; Yoneda, Y.; Tsukihara, T. A high-resolution structure of the pre-microRNA nuclear export machinery. Science 2009, 326, 1275–1279.
- Zhang, H.; Kolb, F.A.; Jaskiewicz, L.; Westhof, E.; Filipowicz, W. Single processing center models for human Dicer and bacterial RNase III. Cell 2004, 118, 57–68.
- Yoda, M.; Kawamata, T.; Paroo, Z.; Ye, X.; Iwasaki, S.; Liu, Q.; Tomari, Y. ATP-dependent human RISC assembly pathways. Nat. Struct. Mol. Biol. 2010, 17, 17–23.
- Kawamata, T.; Tomari, Y. Making RISC. Trends Biochem. Sci. 2010, 35, 368–376.
- Huntzinger, E.; Izaurralde, E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011, 12, 99–110.
- Jackson, R.J.; Standart, N. How do microRNAs regulate gene expression? Sci. Stke Signal Transduct. Knowl. Environ. 2007, 2007, re1.
- Forman, J.J.; Legesse-Miller, A.; Coller, H.A. A search for conserved sequences in coding regions reveals that the let-7 microRNA targets Dicer within its coding sequence. Proc. Natl. Acad. Sci. USA 2008, 105, 14879–14884.
- Stavast, C.J.; Erkeland, S.J. The Non-Canonical Aspects of MicroRNAs: Many Roads to Gene Regulation. Cells 2019, 8, 1465.
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383.
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008.
- Vickers, K.C.; Remaley, A.T. Lipid-based carriers of microRNAs and intercellular communication. Curr. Opin. Lipidol. 2012, 23, 91–97.
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659.
- Cui, M.; Wang, H.; Yao, X.; Zhang, D.; Xie, Y.; Cui, R.; Zhang, X. Circulating MicroRNAs in Cancer: Potential and Challenge. Front. Genet. 2019, 10, 626.
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848.
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer 2017, 17, 302–317.
- Guo, Y.; Ji, X.; Liu, J.; Fan, D.; Zhou, Q.; Chen, C.; Wang, W.; Wang, G.; Wang, H.; Yuan, W.; et al. Effects of exosomes on pre-metastatic niche formation in tumors. Mol. Cancer 2019, 18, 124.
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3.
- Gangoda, L.; Boukouris, S.; Liem, M.; Kalra, H.; Mathivanan, S. Extracellular vesicles including exosomes are mediators of signal transduction: Are they protective or pathogenic? Proteomics 2015, 15, 260–271.
- Baroni, S.; Romero-Cordoba, S.; Plantamura, I.; Dugo, M.; D’Ippolito, E.; Cataldo, A.; Cosentino, G.; Angeloni, V.; Rossini, A.; Daidone, M.G.; et al. Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis. 2016, 7, e2312.
- Tian, X.P.; Wang, C.Y.; Jin, X.H.; Li, M.; Wang, F.W.; Huang, W.J.; Yun, J.P.; Xu, R.H.; Cai, Q.Q.; Xie, D. Acidic Microenvironment Up-Regulates Exosomal miR-21 and miR-10b in Early-Stage Hepatocellular Carcinoma to Promote Cancer Cell Proliferation and Metastasis. Mol. Cancer Res. MCR 2019, 9, 1965–1979.
- Bertolini, I.; Storaci, A.M. Interplay Between V-ATPase G1 and Small EV-miRNAs Modulates ERK1/2 Activation in GBM Stem Cells and Nonneoplastic Milieu. Tumor Microenviron. Immunobiol. 2020, 18, 1744–1754.
- Zhang, Y.; Wang, S.; Lai, Q.; Fang, Y.; Wu, C.; Liu, Y.; Li, Q.; Wang, X.; Gu, C.; Chen, J.; et al. Cancer-associated fibroblasts-derived exosomal miR-17-5p promotes colorectal cancer aggressive phenotype by initiating a RUNX3/MYC/TGF-β1 positive feedback loop. Cancer Lett. 2020, 491, 22–35.
- Wang, L.; Yang, G.; Zhao, D.; Wang, J.; Bai, Y.; Peng, Q.; Wang, H.; Fang, R.; Chen, G.; Wang, Z.; et al. CD103-positive CSC exosome promotes EMT of clear cell renal cell carcinoma: Role of remote MiR-19b-3p. Mol. Cancer 2019, 18, 86.
- Guo, L.; Zhu, Y.; Li, L.; Zhou, S.; Yin, G.; Yu, G.; Cui, H. Breast cancer cell-derived exosomal miR-20a-5p promotes the proliferation and differentiation of osteoclasts by targeting SRCIN1. Cancer Med. 2019, 8, 5687–5701.
- Zhao, Q.; Liu, C.; Xie, Y.; Tang, M.; Luo, G.; Chen, X.; Tian, L.; Yu, X. Lung Cancer Cells Derived Circulating miR-21 Promotes Differentiation of Monocytes into Osteoclasts. OncoTargets Ther. 2020, 13, 2643–2656.
- Fabbri, M.; Paone, A.; Calore, F.; Galli, R.; Gaudio, E.; Santhanam, R.; Lovat, F.; Fadda, P.; Mao, C.; Nuovo, G.J.; et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. J. Cell. Physiol. 2012, 109, E2110–E2116.
- Yuan, X.; Qian, N.; Ling, S.; Li, Y.; Sun, W.; Li, J.; Du, R.; Zhong, G.; Liu, C.; Yu, G.; et al. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Breast Cancer Res. BCR 2021, 11, 1429–1445.
- Donnarumma, E.; Fiore, D.; Nappa, M.; Roscigno, G.; Adamo, A.; Iaboni, M.; Russo, V.; Affinito, A.; Puoti, I.; Quintavalle, C.; et al. Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer. Oncotarget 2017, 8, 19592–19608.
- Lin, F.; Yin, H.B.; Li, X.Y.; Zhu, G.M.; He, W.Y.; Gou, X. Bladder cancer cell‑secreted exosomal miR‑21 activates the PI3K/AKT pathway in macrophages to promote cancer progression. Int. J. Oncol. 2020, 56, 151–164.
- Lan, J.; Sun, L.; Xu, F.; Liu, L.; Hu, F.; Song, D.; Hou, Z.; Wu, W.; Luo, X.; Wang, J.; et al. M2 Macrophage-Derived Exosomes Promote Cell Migration and Invasion in Colon Cancer. Cancer Res. 2019, 79, 146–158.
- Zeng, Z.; Li, Y.; Pan, Y.; Lan, X.; Song, F.; Sun, J.; Zhou, K.; Liu, X.; Ren, X.; Wang, F.; et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat. Commun. 2018, 9, 5395.
- Fang, J.H.; Zhang, Z.J.; Shang, L.R.; Luo, Y.W.; Lin, Y.F.; Yuan, Y.; Zhuang, S.M. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology 2018, 68, 1459–1475.
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.; Chin, A.R.; et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014, 25, 501–515.
- Fukuda, T.; Yao, K.; Kanda, H.; Ae, K.; Okawa, A.; Akazawa, C.; Ochiya, T.; Futakuchi, M.; Takeda, S.; Sato, S.; et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Proc. Natl. Acad. Sci. USA 2015, 17, 183–194.
- Zhang, Y.; Meng, W.; Yue, P.; Li, X. M2 macrophage-derived extracellular vesicles promote gastric cancer progression via a microRNA-130b-3p/MLL3/GRHL2 signaling cascade. J. Exp. Clin. Cancer Res. CR 2020, 39, 134.
- Kosaka, N.; Iguchi, H.; Hagiwara, K.; Yoshioka, Y.; Takeshita, F.; Ochiya, T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J. Biol. Chem. 2013, 288, 10849–10859.
- Jung, K.O.; Youn, H.; Lee, C.H.; Kang, K.W.; Chung, J.K. Visualization of exosome-mediated miR-210 transfer from hypoxic tumor cells. Oncotarget 2017, 8, 9899–9910.
- Lin, X.J.; Fang, J.H.; Yang, X.J.; Zhang, C.; Yuan, Y.; Zheng, L.; Zhuang, S.M. Hepatocellular Carcinoma Cell-Secreted Exosomal MicroRNA-210 Promotes Angiogenesis In Vitro and In Vivo. Mol. Ther. Nucleic Acids 2018, 11, 243–252.
- Dror, S.; Sander, L.; Schwartz, H.; Sheinboim, D.; Barzilai, A.; Dishon, Y.; Apcher, S.; Golan, T.; Greenberger, S.; Barshack, I.; et al. Melanoma miRNA trafficking controls tumour primary niche formation. Nat. Cell Biol. 2016, 18, 1006–1017.
- Yin, Y.; Cai, X.; Chen, X.; Liang, H.; Zhang, Y.; Li, J.; Wang, Z.; Chen, X.; Zhang, W.; Yokoyama, S.; et al. Tumor-secreted miR-214 induces regulatory T cells: A major link between immune evasion and tumor growth. Cell Res. 2014, 24, 1164–1180.
- Liu, X.; Cao, M.; Palomares, M.; Wu, X.; Li, A.; Yan, W.; Fong, M.Y.; Chan, W.C.; Wang, S.E. Metastatic breast cancer cells overexpress and secrete miR-218 to regulate type I collagen deposition by osteoblasts. Cancer Med. 2018, 20, 127.
- Yang, M.; Chen, J.; Su, F.; Yu, B.; Su, F.; Lin, L.; Liu, Y.; Huang, J.D.; Song, E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol. Cancer 2011, 10, 117.
- Wang, X.; Luo, G.; Zhang, K.; Cao, J.; Huang, C.; Jiang, T.; Liu, B.; Su, L.; Qiu, Z. Hypoxic Tumor-Derived Exosomal miR-301a Mediates M2 Macrophage Polarization via PTEN/PI3Kgamma to Promote Pancreatic Cancer Metastasis. Cancer Res. 2018, 78, 4586–4598.
- Sakha, S.; Muramatsu, T.; Ueda, K.; Inazawa, J. Exosomal microRNA miR-1246 induces cell motility and invasion through the regulation of DENND2D in oral squamous cell carcinoma. Sci. Rep. 2016, 6, 38750.
- Yin, Z.; Ma, T.; Huang, B.; Lin, L.; Zhou, Y.; Yan, J.; Zou, Y.; Chen, S. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-β signaling pathway. J. Exp. Clin. Cancer Res. CR 2019, 38, 310.
- Zhao, S.; Mi, Y.; Guan, B.; Zheng, B.; Wei, P.; Gu, Y.; Zhang, Z.; Cai, S.; Xu, Y.; Li, X.; et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J. Hematol. Oncol. 2020, 13, 156.
- Di Modica, M.; Regondi, V.; Sandri, M.; Iorio, M.V.; Zanetti, A.; Tagliabue, E.; Casalini, P.; Triulzi, T. Breast cancer-secreted miR-939 downregulates VE-cadherin and destroys the barrier function of endothelial monolayers. Cancer Lett. 2017, 384, 94–100.
- Hashimoto, K.; Ochi, H.; Sunamura, S.; Kosaka, N.; Mabuchi, Y. Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A. Oncotargets Ther. 2018, 115, 2204–2209.
- Fang, T.; Lv, H.; Lv, G.; Li, T.; Wang, C.; Han, Q.; Yu, L.; Su, B.; Guo, L.; Huang, S.; et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat. Commun. 2018, 9, 191.
- Koren, E.; Fuchs, Y. The bad seed: Cancer stem cells in tumor development and resistance. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 2016, 28, 1–12.
- Dimou, A.; Syrigos, K.N.; Saif, M.W. Overcoming the stromal barrier: Technologies to optimize drug delivery in pancreatic cancer. Ther. Adv. Med Oncol. 2012, 4, 271–279.
- Lawson, D.A.; Bhakta, N.R.; Kessenbrock, K.; Prummel, K.D.; Yu, Y.; Takai, K.; Zhou, A.; Eyob, H.; Balakrishnan, S.; Wang, C.Y.; et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature 2015, 526, 131–135.
- Charafe-Jauffret, E.; Ginestier, C.; Iovino, F.; Wicinski, J.; Cervera, N.; Finetti, P.; Hur, M.H.; Diebel, M.E.; Monville, F.; Dutcher, J.; et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009, 69, 1302–1313.
- Pang, R.; Law, W.L.; Chu, A.C.; Poon, J.T.; Lam, C.S.; Chow, A.K.; Ng, L.; Cheung, L.W.; Lan, X.R.; Lan, H.Y.; et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell 2010, 6, 603–615.
- Marcato, P.; Dean, C.A.; Pan, D.; Araslanova, R.; Gillis, M.; Joshi, M.; Helyer, L.; Pan, L.; Leidal, A.; Gujar, S.; et al. Aldehyde dehydrogenase activity of breast cancer stem cells is primarily due to isoform ALDH1A3 and its expression is predictive of metastasis. Stem Cells 2011, 29, 32–45.
- Kingsley, L.A.; Fournier, P.G.; Chirgwin, J.M.; Guise, T.A. Molecular biology of bone metastasis. Mol. Cancer Ther. 2007, 6, 2609–2617.
- Ping, Q.; Yan, R.; Cheng, X.; Wang, W.; Zhong, Y.; Hou, Z.; Shi, Y.; Wang, C. Cancer-associated fibroblasts: Overview, progress, challenges, and directions. Cancer. Gene. Ther. 2021.
- Shoucair, I.; Weber Mello, F. The Role of Cancer-Associated Fibroblasts and Extracellular Vesicles in Tumorigenesis. Cancer Gene Ther. 2020, 21, 6837.
- Mitra, A.K.; Zillhardt, M.; Hua, Y.; Tiwari, P.; Murmann, A.E.; Peter, M.E.; Lengyel, E. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov. 2012, 2, 1100–1108.
- Tang, X.; Hou, Y.; Yang, G.; Wang, X.; Tang, S.; Du, Y.E.; Yang, L.; Yu, T.; Zhang, H.; Zhou, M.; et al. Stromal miR-200s contribute to breast cancer cell invasion through CAF activation and ECM remodeling. Cell Death Differ. 2016, 23, 132–145.
- Du, Y.E.; Tu, G.; Yang, G.; Li, G.; Yang, D.; Lang, L.; Xi, L.; Sun, K.; Chen, Y.; Shu, K.; et al. MiR-205/YAP1 in Activated Fibroblasts of Breast Tumor Promotes VEGF-independent Angiogenesis through STAT3 Signaling. Theranostics 2017, 7, 3972–3988.
- Martin, T.A. The role of tight junctions in cancer metastasis. Semin. Cell Dev. Biol. 2014, 36, 224–231.
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887.
- Parisi, L.; Gini, E. Macrophage Polarization in Chronic Inflammatory Diseases: Killers or Builders? J. Immunol. Res. 2018, 2018, 8917804.
- Mortara, L.; Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. J. Immunol. Res. 2020, 877, 173090.
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440.
- Sánchez-González, I.; Bobien, A.; Molnar, C.; Schmid, S.; Strotbek, M.; Boerries, M. miR-149 Suppresses Breast Cancer Metastasis by Blocking Paracrine Interactions with Macrophages. Cancer Res. 2020, 80, 1330–1341.
- Ke, M.; Zhang, Z.; Cong, L.; Zhao, S.; Li, Y.; Wang, X.; Lv, Y.; Zhu, Y.; Dong, J. MicroRNA-148b-colony-stimulating factor-1 signaling-induced tumor-associated macrophage infiltration promotes hepatocellular carcinoma metastasis. Cancer Res. 2019, 120, 109523.
- Chen, E.B.; Zhou, Z.J.; Xiao, K.; Zhu, G.Q.; Yang, Y.; Wang, B.; Zhou, S.L.; Chen, Q.; Yin, D.; Wang, Z.; et al. The miR-561-5p/CX(3)CL1 Signaling Axis Regulates Pulmonary Metastasis in Hepatocellular Carcinoma Involving CX(3)CR1(+) Natural Killer Cells Infiltration. Theranostics 2019, 9, 4779–4794.
