Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Nora Tang and Version 1 by Rambod Abiri.
Organogenesis and somatic embryogenesis are the two substitute pathways in plant micropropagation [16]. However, some literature emphasised the shoot proliferation as a separate class of Eucalyptus micropropagation method. To better understand the prospects of in vitro scenarios, in the coming section, we briefly describe the concepts of organogenesis and somatic embryogenesis in Eucalyptus.
- Eucalyptus
- somatic embryogenesis
- organogenesis
1. Introduction
Eucalyptus (family Myrtaceae) is a large genus of fast-growing evergreen trees and ornamental shrubs with more than 900 species, native to Australia, Indonesia, India, Portugal, South Africa, Brazil, Chile, and France [1]. The economic importance and commercial value of Eucalyptus have long been considered in many parts of the world due to its hard timber, resistance to biotic stresses, rapid growth, and high profitability [2,3,4][2][3][4].
Furthermore, increasing global demand for timber, pulp, and paper products has significantly changed the plantation culture of Eucalyptus species all over the world [5]. Besides having extensive use of Eucalyptus in agroforestry systems, the essential oils extracted from this tree have been widely used in modern ethnopharmaceutical studies due to its various biological activities and medicinal properties [6]. To date, natural and cultivated Eucalyptus forests have been considered as the initial genetic resources for agroforestry plans and tree breeding programs [7]. Despite the abundance of natural Eucalyptus forests, cultivation of Eucalyptus by seed is the most traditional way of propagation with varying degrees of competitive success and establishment. In the latter stage, vegetative propagations methods have also been applied for Eucalyptus improvement programs [8,9][8][9]. Vegetative progeny methods are broadly being used for the asexual propagation of forest trees. The primary vegetative propagation techniques are grafting, layering, root cuttings, and rooting of the shoot. Generally, the effectiveness of the methods depends on the physiological structure, shape, performance, and age of the donor tree [10]. Over recent decades, conventional breeding tools which have been implemented in forestry improvement programs have become the main strategy to multiply forest trees [11]. However, the bottlenecks of conventional breeding techniques opened a new window to the clonal propagation of Eucalyptus.
Clonal propagation is often considered as one of the most critical in vitro platforms to increase the multiplication rate of Eucalyptus sp. in forestry breeding programs and biotechnology research [12]. Micropropagation, which is mainly achieved via somatic embryogenesis and organogenesis, is the true-to-type, virus-free, and rapid propagation technique [13]. The in vitro culture of Eucalyptus depends on several factors including external aspects (sugar concentrations, pH, media composition, plant growth regulators (PGRs), luminosity, and temperature) and internal features (physiological conditions, age, and genotype) [13]. Despite the advancement in the micropropagation method, the obstacles in callus induction, regeneration, root induction, and acclimatisation are still the major challenges for clonal propagation of Eucalyptus. On the other hand, hyperhydricity, rapid browning, and poor explant response, which cause poor growth of explants and even failure of tissue culture procedure, are some of the other major factors affecting in vitro propagation of Eucalyptus [14]. The advantages and disadvantages of micropropagation methods in plants are presented in Figure 1 [15].
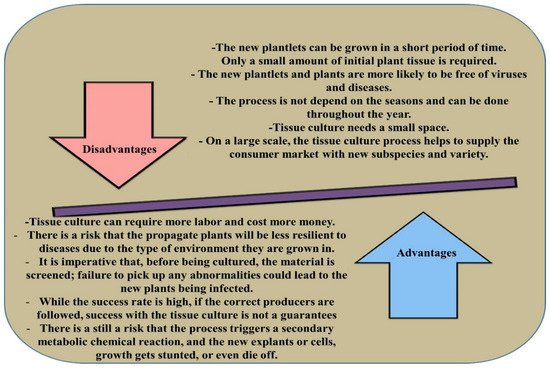
Figure 1. The advantages and disadvantages of micropropagation in plants.
To date, several micropropagation techniques and in vitro protocols have been developed for tissue culture of Eucalyptus. Regardless of the advances, micropropagation of Eucalyptus has been met with limited success (failures) and applicability. This is due to the lack of studies focused on the mechanisms, strategies, and interactions of internal and external factors under in vitro conditions. Keeping this in view, this current review aims to clarify the rationale mechanisms behind the observed phenomenon in Eucalyptus tissue culture. Furthermore, this is an extensive review of some intriguing aspects of internal mechanisms of Eucalyptus in response to external (Physico-chemical) factors under in vitro conditions.
2. Organogenesis
Organogenesis is the vegetative propagation by which plant organs such as flower buds, shoots, and roots are produced from the cells and tissues (the unusual points of origin) under in vitro conditions. The type of organogenesis (direct and indirect) depends on the presence of relative concentrations of hormones and explants in the culture medium [17][16]. Indirect organogenesis is the formation and development of organs from an amorphous tissue, callus, or cell suspension. Additionally, this propagation method is a reliable technique, which has been applied for the production of genetically modified plants from calli [18][17]. On the other hand, the development of new organs from the explants (axillary buds), which forms new shoots and buds, is known as direct organogenesis [19][18].
In Eucalyptus, the composition of different factors such as carbon, various vitamins, amino acids, gelling agent, macro-and micronutrients, and other additives has affected the efficacy of both types of organogenesis [20][19]. For instance, during indirect organogenesis of Eucalyptus camaldulensis hybrids, the combination of naphthyl acetic acid (NAA) and 6-benzylaminopurine (BAP) showed a better efficacy as compared to the application of zeatin, kinetin, casein hydrolysate, and 2,4-Dichlorophenoxyacetic acid (2,4-D) [21][20]. Additionally, callus induction was observed in E. camaldulensis hybrids cultivated on Murashige and Skoog Basal (MS) media added with 1 mg L−1 NAA, and the highest rate of somatic embryogenesis was achieved on MS basal medium fortified with 0.1 mg l−1 NAA and 0.5 mg l−1 benzyladenine (BA) [22][21].
3. Somatic Embryogenesis
Somatic embryogenesis (SE) is a multifactorial, non-sexual, and complex biotechnological tool. This artificial method produces bipolar embryos through physiological, biochemical, and molecular pathways from somatic tissues. During SE, somatic cells of plants become totipotent and alter their pathway of development, resulting in the formation of a complete plant from somatic embryos [23][22]. In plants, two arrays of the somatic embryos have been reported including indirect somatic embryogenesis induced from an unorganised callus and direct somatic embryogenesis induced from the pre-embryogenic cells (cells of the nucellus) [24][23]. Indirect embryogenesis needs redetermination of differentiated cells process, callus induction, proliferation, and the development of the embryogenically determined phase [25][24]. Generally, the effects of suitable PGRs at specific concentrations play an integral role in the re-entry of cells into mitosis and determination of the embryogenic phase [26][25]. Nonetheless, during direct somatic embryogenesis, which is a rare type of SE, the cell(s) produces embryos without the formation of an intervening callus. To achieve direct embryogenesis, pre-embryogenic determined cells (PEDC) require favourable conditions and specific type of PGRs to enter mitosis and complete embryogenesis [27][26].
Over the decades, numerous somatic embryogenesis protocols for several Eucalyptus species have been reported [13,28,29][13][27][28]. However, the occurrence of somaclonal variation, low percentage of embryogenic initiation, and an inability of somatic embryos to reach complete maturation limit somatic embryogenesis adoption for clonal propagation in Eucalyptus [14]. It has been also reported that several factors, such as hormonal type and level, culture media, and ontogenetic age of tissue interfere with the diverse phases of somatic embryos [30][29]. Nonetheless, there is a notorious paucity of studies addressing ultrastructural, histological, and cytological evidence on the diverse features linked to the frequency induction and proliferation of somatic embryos. Despite the morphological resemblances of somatic embryos with zygotic ones at the proliferation and developmental phases, a lack of clear definition has been stated in some other phases of proliferation and developmental steps in Eucalyptus [31][30]. For example, histological and morphological investigations on the embryogenic cell of Eucalyptus globulus, Eucalyptus grandis, and Eucalyptus nitens at diverse development phases demonstrated the usual features of other somatic embryogenic structures including small vacuole, prominent nucleus, small volume, and dense cytoplasm [32][31].
The success rate of a propagation technique in Eucalyptus depends on both quality (genetic stability and growth rate) and quantity (survival) of the regenerated plants. The combination of the above-mentioned factors associated with the genotypes of Eucalyptus is the most effective aspect in both somatic embryogenesis and organogenesis. In the following sections, the effects of the most important factors on both somatic embryogenesis and organogenesis of Eucalyptus are discussed.
References
- Batista, T.R.; Mendonase, E.G.; Padua, M.S.; Stein, V.C.; Paiva, L. Morpho and cytological differentiation of calli of Eucalyptus grandis x Eucalyptus urophylla during somatic embryogenesis. Braz. Arch. Biol. Technol. 2018, 61, 1–11.
- Merkle, S.A.; Nairn, C.J. Hardwood tree biotechnology. In Vitro Cell. Dev. Biol. Plant. 2005, 41, 602–619.
- Nogueira, M.C.D.J.A.; de Araujo, V.A.; Vasconcelos, J.S.; Christoforo, A.L.; Lahr, F.A.R. Sixteen properties of Eucalyptus Tereticornis wood for structural uses. Bioscience 2020, 36, 449–457.
- Rezende, G.D.S.; de Resende, M.D.V.; de Assis, T.F. Eucalyptus breeding for clonal forestry. In Challenges and Opportunities for the World’s Forests in the 21st Century; Springer: Berlin/Heidelberg, Germany, 2014; pp. 393–424.
- Benra, F.; Nahuelhual, L.; Gaglio, M.; Gissi, E.; Aguayo, M.; Jullian, C.; Bonn, A. Ecosystem services tradeoffs arising from non-native tree plantation expansion in southern Chile. Landsc. Urban Plan. 2019, 190, 1–19.
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848.
- Hirsch, H.; Allsopp, M.H.; Canavan, S.; Cheek, M.; Geerts, S.; Geldenhuys, C.J.; Harding, G.; Hurley, B.P.; Jones, W.; Keet, J.H. Eucalyptus camaldulensis in South Africa- “past, present, future”. Trans. R. Soc. S. Afr. 2020, 75, 1–22.
- Kerk, N.M.; Jiang, K.; Feldman, L.J. Auxin metabolism in the root apical meristem. Plant Physiol. 2000, 122, 925–932.
- Stokes, A.; Atger, C.; Bengough, A.G.; Fourcaud, T.; Sidle, R.C. Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant Soil 2009, 324, 1–30.
- Monteuuis, O. Vegetatively propagating forest trees. In Proceedings of the Fourth International Conference of the IUFRO Unit 2.09.02 on “Development and Application of Vegetative Prpagation Technologiesd in Plantation Forestry Cope with a Changing Climate and Environemnt”, La Plata, Argentina, 19–23 September 2016; pp. 37–57.
- Naidoo, S.; Slippers, B.; Plett, J.M.; Coles, D.; Oates, C.N. The road to resistance in forest trees. Front. Plant Sci. 2019, 10, 273.
- Nakhooda, M.; Watt, M.P.; Mycock, D. The choice of auxin analogue for in vitro root induction influences post-induction root development in Eucalyptus grandis. Turk. J. Agric. For. 2014, 38, 258–266.
- Pinto, G.; Park, Y.; Neves, L.; Araujo, C.; Santos, C. Genetic control of somatic embryogenesis induction in Eucalyptus globulus Labill. Plant Cell Rep. 2008, 27, 1093–1101.
- Jain, S.M. An updated overview of advances in somatic embryogenesis in forest trees. In Plantation Technology in Tropical Forest Science; Springer: Berlin/Heidelberg, Germany, 2006; pp. 113–122.
- Hussain, A.; Qarshi, I.A.; Nazir, H.; Ullah, I. Plant Tissue Culture: Current Status and Opportunities. Recent Advances in Plant In Vitro Culture; Leva, A., Ed.; InTech: London, UK, 2012; pp. 1–28.
- Stewart, C.N., Jr. Plant Biotechnology and Genetics: Principles, Techniques, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016.
- Johns, A.E. Lessons for Plant Micropropagation; Educreation Publishing: Chhattisgarh, India, 2019; p. 85.
- Trueman, S.J.; Hung, C.D.; Wendling, I. Tissue culture of Corymbia and Eucalyptus. Forests 2018, 9, 84.
- Kendurkar, S.V.; Rangaswamy, M. in vitro Approaches for the Improvement of Eucalyptus. In Biotechnologies of Crop Improvement; Springer: Berlin/Heidelberg, Germany, 2018; pp. 159–214.
- Arya, I.; Chauhan, S.S.S.; Arya, S. Micropropagation of superior Eucalyptus hybrids FRI-5 (Eucalyptus camaldulensis Dehn x E. tereticornis Sm) and FRI-14 (Eucalyptus torelliana FV Muell x E. citriodora Hook): A commercial multiplication and field evaluation. Afr. J. Biotechnol. 2009, 8, 5718–5726.
- Prakash, M.; Gurumurthi, K. Effects of type of explant and age, plant growth regulators and medium strength on somatic embryogenesis and plant regeneration in Eucalyptus camaldulensis. Plant Cell Tissue Organ Cult. 2010, 100, 13–20.
- Guerra, M.; Fraga, H.; Vieira, L.; Ree, J.; Heringer, A.; Maldonado, S.B. Fundamentals, advances and applications of somatic embryogenesis in selected Brazilian native species. Acta Hortic. 2016, 1113, 1–12.
- Kundu, S.; Gantait, S. Fundamental facets of somatic embryogenesis and its applications for advancement of peanut biotechnology. In Biotechnologies of Crop Improvement; Springer: Berlin/Heidelberg, Germany, 2018; pp. 267–298.
- Nazir, M.; Sadat, S.; Soltani Howyzeh, M. The effect of different hormone combinations on direct and indirect somatic embryogenesis in Agave americana. Plant Physiol. 2019, 9, 2739–2747.
- Ochatt, S.J.; Abirached-Darmency, M. The underlying processes governing seed size plasticity: Impact of endoploidy on seed coat development and cell expansion in Medicago truncatula. In The Model Legume Medicago truncatula, 1st ed.; de Bruijn, F.J., Ed.; John Wiley & Sons, Inc.: London, UK, 2020; pp. 99–115.
- Elmeer, K.E.S. Factors regulating somatic embryogenesis in plants. In Somatic Embryogenesis and Gene Expression; Narosa Publishing House: New Delhi, India, 2013; pp. 56–81.
- Martinez, M.T.; San Jose, M.D.C.; Arrillaga, I.; Cano, V.; Morcillo, M.; Cernadas, M.J.; Corredoira, E. Holm oak somatic embryogenesis: Current status and future perspectives. Front. Plant Sci. 2019, 10, 1–14.
- Lelu-Walter, M.A.; Thompson, D.; Harvengt, L.; Sanchez, L.; Toribio, M.; Paques, L.E. Somatic embryogenesis in forestry with a focus on Europe: State-of-the-art, benefits, challenges and future direction. Tree Genet. Genom. 2013, 9, 883–899.
- Moura, L.C.D.; Xavier, A.; Cruz, A.C.F.D.; Gallo, R.; Gatti, K.C.; Miranda, N.A.; Otoni, W.C. Effects of explant type, culture media and picloram and dicamba growth regulators on induction and proliferation of somatic embryos in Eucalyptus grandis x E urophylla1. Rev. Árvore 2017, 41, 1–10.
- Carraro, N.; Tisdale-Orr, T.E.; Clouse, R.M.; Knoller, A.S.; Spicer, R. Diversification and expression of the PIN, AUX/LAX, and ABCB families of putative auxin transporters in Populus. Front. Plant Sci. 2012, 3, 17.
- Pinto, G.; Park, Y.S.; Loureiro, J.; Neves, L.; Araujo, C.; Silva, S.; Santos, C. Somatic embryogenesis in Eucalyptus -an update to 2009. Appl. Plant Biotechnol. In Vitro Propagat. Plant Transf. Second. Metabol. Prod. 2009, 1, 531–542.
More
