Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Antonio Pizzi and Version 2 by Vivi Li.
The origin of tannins, their historical evolution, their different types, and their applications are described. Old and established applications are described, as well as the future applications which are being developed at present and that promise to have an industrial impact in the future. The chemistry of some of these applications is discussed where it is essential to understand the tannins and their derivates role. The essential points of each application, their drawbacks, and their chance of industrial application are briefly discussed.
- tannins
- applications
- new applications
- drawbacks
- advantages
1. Origin of Tannins
Leather tanning has been used for centuries, even millennia, by immersing skins in water where special barks or woods containing tannin have been added. Up to a full year was necessary for leather to be produced in such a manner. However, the current tannin extraction industry is relatively newer. The more modern history of tannins began in the 17th century when Giovannetti, an Italian chemist, studied the interactions between iron solutions and substances called “astringents”. In 1772, various researchers identified the presence of an acid in these compounds. This acid was then isolated by Scheele and turned out to be gallic acid. Based on the experiments of Deyeux and Bartholdi, continued by Proust in the late 18th and early 19th centuries, tannins have been officially recognized as a discrete group of different molecules based on gallic acid content. The great growth of the tannin extraction industry began in the years around 1850 in Lyon, where tannin was used as iron tannate for the black coloring of silk for women’s blouses [1]. After 10 years, fashion changed and thus after many bankruptcies and groupings of factories, tannin manufacturers were able to convince the leather industry to use tannin in place of oak chips with considerable savings in tanning time (from 12 months with the old system based on wood chips rich in tannin to 28 days using tannin extract) [1]. The benefits of tannin extracts in the manufacture of leather, and even the time savings still allowed by their use, were such that the industry expanded rapidly and thrived. Tannin being in short supply in Europe, factories were opened in distant countries to satisfy the growing demand and promoting the use of alternative tannin types. Early in the 20th century, South American and southern and central African tannins began to be industrially extracted to supply major markets in Europe and North America. Among these, the main ones were quebracho wood and mimosa bark tannins. Leather processing was thus the second major boom period for the tannin industry. After World War II the substitution of leather with synthetic materials for shoes again caused a number of tannin extraction plants to close [2]. The third period of use of tannin therefore began, first with their development as bio-based adhesives and later with an increasing number of applications in new bio-based materials, this latter period being still in full development.
The name “tannin” comes from the use of this class of compounds in the tanning process of hides to give leather. Their general appearance varies, ranging from white amorphous powders to off-white amorphous powders, to glossy, almost colorless pasty substances, to reddish-brown powders when produced by spray drying. They have an astringent taste. Tannins are natural products found in most higher plants. They are produced in almost all parts of the plant, namely seeds, roots, bark, wood, and leaves, because of their fundamental role in the defense of the plant against insects, food infections, fungi, or bacteria. The defense mechanism is based on the ability of tannins to complex proteins irreversibly. They are also considered as one of the effective components contributing to the fact that the risk of suffering from cardiovascular diseases and some forms of cancer can be reduced by choosing diets rich in fruits and vegetables. In addition to their documented effects on human health, tannins are also important for the welfare of ruminants; high protein feeds such as alfalfa trigger the production in the rumen of methane trapped as proteinaceous foam, resulting in a potentially mortal fermentation that can be reduced by adding tannin in the diet. Two wide classes of tannins exist: hydrolysable tannins such as gallo-tannins and ellagi-tannins, and condensed polyflavonoid tannins, these latter being stable and rarely subject to hydrolysis [2].
2. Industrial Utilization
The industrial uses briefly described below are in order of present or probable future importance to give an idea of what is developed and used already, and which applications are likely to gather importance in the future.2.1. Leather Tanning
The manufacture of leather is still the largest use of tannins of vegetable origin. Leather has traditionally been made in ground pits in which alternating layers of animal skins and wood chips containing tannins, such as oak chips, have been placed and soaked for considerable periods of time. The skins were passed through a number of consecutive pits, generally 28, so as to be slowly enriched and “tanned” by the tannin in solution. The tannins exfiltrated the wood chips and slowly impregnated increasingly more of each skin. Such a manufacturing system was practiced for many centuries and produced good quality leather but it took several months, often a whole year, for the leather to be ready. The first change came when the tannin extraction industry, which had grown considerably in the 1850s to supply black iron tannate dyes to color silk, was in a desperate position because of the change in fashion. Some tannin extraction plants were able to demonstrate that by directly adding tannin extract to traditional tanning pits, the same quality of leather could be produced in just 28 days (28 pits, one per day). Leather tanned in this way began to prevail and this use led to a boom which lasted mainly until the end of the Second World War. In particular, the war years were good simply because armies were walking in leather boots.
At the end of the Second World War, three events contributed significantly to the steady decline in vegetable tanned leather. First, the introduction of synthetic materials, derived from petrol, for shoe soles begun to compete strongly with leather for one of its most traditional applications. Secondly, the demobilization of armies, which sharply reduced the need for leather shoes, and the third, the strong penetration of the market by chromium salt tanning for the manufacture of soft leathers, in particular the upper part of shoes. With all these changes, while vegetable leather has now begun to gain a reputation as a luxury product, there are still some important niches for which it is used such as equestrian equipment, heavy bags and luggage, and other heavy applications as well as the real high price luxury markets. Although the traditional 28 pits tanning method still exists for a number of special leathers as well as in the case of artisanal leather (as in Morocco for example), the tanning processes has also evolved for vegetable tanning where rotary drums, a technique borrowed from chrome tanning, allows vegetable tanning to be finalized in about 24 h. Today, research on vegetable tanned leather has been able to produce much more supple leather through the inclusion of oils and other techniques, so that some rebirth of the use of plant tannins for other application areas appears to take place.
2.2. Wood Adhesives
There are a number of detailed reviews on the use of tannins for wood adhesives. The reader is referred to these detailed studies [2][3][2,10]. However, here existing technologies and industrial use of wood tannin adhesives are presented.
As extensive studies already exist, and this application of tannin is now the second most important after leather manufacturing, only a few of the main achievements of tannin-based adhesives for wood products will be highlighted. (1) The development, optimization, and industrialization of non-fortified but chemically modified thermosetting tannins for particleboard, other particle products, and plywood [4][5][6][11,12,13]. (2) The technology for rapidly pressing tannin adhesives for particle board, which is also industrial [7][8][14,15]. (3) The development and industrialization of tannin–urea–formaldehyde adhesives for plywood and in particular as impregnators for corrugated board starch binders [9][16]. (4) The development and industrialization of cold-setting tannin–resorcinol–formaldehyde adhesives for glulam and fingerjointing [10][17]. (5) The large-scale development and industrialization of fast-setting “honeymoon” separate application cold-setting adhesives for tannin-bonded glulam and fingerjoints [11][12][13][18,19,20] (Figure 14).


Figure 14. Development of strength visualized through the rapid increase in percentage wood failure of tannin-based fast-setting “honeymoon” separate application cold-setting adhesives for glulam and fingerjoints.
(6) The development and industrialization of zinc salts to accelerate the hardening of non-fortified tannin adhesives for plywood [2][14][15][16][2,21,22,23]. (7) Successful formulation, development, and industrialization in Chile of pine bark tannin adhesives for particle boards and for glulam and fingerjointing [6][17][13,24]. (8) The development of isocyanate/tannin copolymers as difficult-to-bond hardwood adhesives and for plywood and other applications [18][19][25,26]. (9) The development of very low formaldehyde tannin adhesives for particle boards and other wood panels. (10) The development of the use of hardeners other than formaldehyde for thermosetting tannin adhesives [2][20][21][2,27,28]. (11) The discovery and development of self-condensation of tannin for adhesives [22][23][24][25][26][27][28][29][29,30,31,32,33,34,35,36].
All industrialized technologies today are based on paraformaldehyde or hexamethylene tetramine (hexamine) [29][36]. The latter is much more user and environmentally friendly.
As regards wood adhesives, a number of experimental improvements have been studied, dictated by the new environment in which wood adhesives must operate. First of all, the relative scarcity of tannins produced in the world, compared to the tonnage of synthetic adhesives used in the panel industry, has led to a great deal of research on the extension of the tannin resource in order to have larger tonnage. As the potential material for tannin extraction shows that millions of tons of this material can be extracted each year worldwide, some companies have started to build additional extraction plants. This movement is still relatively small, but it is ongoing. The second approach, to extend the tannin with another abundant and natural material, has led to the preparation of adhesives based on in situ copolymers of tannins and lignin [30][37] or copolymers of tannin and protein or soy flour [31][38], and the use of tannin–furfuryl alcohol adhesive formulations, furfuryl alcohol being also a bio-based material [32][39].
The second new constraint is the demand of most companies to eliminate formaldehyde emissions from tannin adhesive. This quest has taken two approaches: (1) total elimination of formaldehyde by substituting it with aldehydes, which are less or non-toxic, and non-volatile [21][33][28,40], such as glyoxal, glutaraldehyde, or vanillin, the latter giving a fully bio-based tannin adhesive, and even aldehydes generated by the action of sodium periodate on glucose, sucrose and even oligomeric carbohydrates, (2) the use of non-aldehyde hardeners such as trishydroxymethylnitromethane [34][41] and trishydroxymethylaminomethane [35][42] or even by combination with furfuryl alcohol, the latter functioning both as a hardener and a contributor to a tannin/furan copolymer [32][36][39,43]. (3) The use of hexamine with the formation of –CH2–NH–CH2– bridges between the tannin molecules, where the secondary amine is capable of absorbing any emission of formaldehyde from the heating of the wood itself or any other emission of formaldehyde to produce truly zero-formaldehyde emission panels [29][37][38][39][36,44,45,46]. (4) Lastly, the hardening of the tannins by autocondensation without the addition of a hardener, autocondensation catalysed by the wood substrate itself in the case of fast-acting procyanidin tannins, such as pine bark tannins, and for slower tannins by addition of silica or silicate or other accelerators [3][22][23][24][25][26][27][28][10,29,30,31,32,33,34,35] allowing the preparation of wood particleboard of indoor quality.
2.3. Pharmaceutical and Medical Applications
Tannins are known bactericides because they react with proteins irreversibly, thus complexing within bacterial membranes, neutralizing their activity. As a consequence, tannin-based pharmaceuticals to cure intestine infections have long-time been marketed. They have effective anticaries properties. Tannins have also many applications for other pharmaceutical/medical uses but all these are targeted for future use rather than the present.
Several experimental studies on the pharmaceutical use of tannins have been published with antitumor and anti-oncogenic activities particularly well documented [40][41][42][43][44][45][47,48,49,50,51,52]. Their antiviral effectiveness is also well documented by in vitro screening for a variety of 12 different hydrolysable and condensed tannins [44][51]. The tannin’s minimal inhibitory concentration (MIC) needed for reducing by 50% the cytopathogenicity induced by a number of viruses was used as an evaluation of their effectiveness. The lower MIC values yielded the best antiviral behavior. The different tannin’s minimum cytotoxic concentration (MCC) needed to detect microscopic alteration of normal cell morphology was also determined. Less toxic is the tannin tested in the patient’s cells, thus the higher is its MCC value, the more acceptable it is as an antiviral compound. The ideal antiviral compound is then the one presenting a combination of the lowest MIC and the highest MCC. The effectiveness of different tannins due to their polyphenolic nature can be very high against a number of different viruses. This is due to their irreversible reaction and combination with the viruses capsid proteins. It is the same reaction used in leather tannins and in their association with carbohydrates.
Thus, a number of commercially available tannins have been tested, namely mimosa bark tannin extract and its derivatives, chestnut tannin extract, tara tannin, quebracho wood tannin extract both sulphited and natural, pecan nut tannin extract, pine bark tannin extract, sumach tannin extract, and spruce tannin extract [44][51]. The viruses against which all these have been tested are highly varied, such as HIV-1 and HIV2, Herpes simplex 1 and 2, Vaccinia virus, vesicular stomatitis virus, Coxsackie virus B4, respiratory syncytial virus, Influenza A H1-N1, Influenza A H3-N2, Influenza B, Human Corona virus, Reovirus-1, Feline Corona virus, Sindbis virus, para-influenza 3 virus, and Punta Toro virus [45][52].
The inhibitory effects of these tannins have also been tested on proliferation of murine leukemia cells, murine mammary carcinoma cells, and human T-cells [44][51].
Acutissimin A is a bound flavonoid with an ellagi-tannin. It is formed by the interaction of a wine flavonoid with the vescalagin generated by the barrel’s oak tannin [46][47][53,54]. Acutissimin A has been found to present an effectiveness 250 times higher to stop tumors growth than the drug Etoposide.
While many studies have been conducted on a variety of tannins derived from a wide variety of plants as an anticancer treatment, some studies on the possibility of using tannin for other medical applications have also been highlighted. Condensed tannins are traditionally used for the treatment of intestinal problems [48][49][55,56]. This is due to their complexation ability with other molecules and their antioxidant behavior. The extract of Stryphnodendron rotundifolium and of other tannins has proven their effectiveness against ulcers by functioning as a protective coating of the gastrointestinal tract [50][51][52][57,58,59]. Other possible mechanisms of action of phenolic plant extracts as herbal medicines against ulcers and gastritis have also been described [50][51][52][53][57,58,59,60].
 Testing on a continuous production line has yielded positive results. This system was tried in plant trials in Switzerland on a continuous production line for polyurethane foam mats where addition of a small amount of isocyanate was necessary as without it the equipment of the factory could not function [61][68].
Testing on a continuous production line has yielded positive results. This system was tried in plant trials in Switzerland on a continuous production line for polyurethane foam mats where addition of a small amount of isocyanate was necessary as without it the equipment of the factory could not function [61][68].
2.4. Wine, Beer, and Fruit Juices Additives and Antioxidants
Wine, beer, and fruit juices naturally contain tannins [54][61]. It is actually their presence that accounts for their characteristic taste. In short, the level of tannin in any of these products must be within a definite interval/concentration range for the beverage to be organoleptically pleasing. Too low an amount of tannin and the beverage will be insipid and with no taste. Too high the proportion of tannins in the beverage and it is too unpleasant, too “tanning” for the consumer’s mouth. Many wines, some beers, and several fruit juices however contain too low a concentration of tannin and thus may need to be “doctored”. Initially, addition of tannin or tannin-rich wood chips directly to wine to enhance its taste and give the impression of a wine of greater age was strictly forbidden in most European countries. With the determined and successful push for wine markets by southern hemisphere producers where limitations on adding oak particles to the wine to accelerate its aging was not forbidden, producers of more established countries tried to defend their market in a different way. Some producers the wines of which were particularly low in tannin content started to add tannin directly to some of their wines. Although addition of additives for aging was legally forbidden the perfectly legal gap existed permitting the use of tannins to precipitate proteic matter in the wine to render it “clearer”. All what was needed then was to add more tannins than what would be required to render the wine “clear”. This was kept fairly confidential, to not incur potential problems. The situation changed dramatically once the so-called “French paradox” came to be known. Namely, notwithstanding that traditional French diet is very rich in fats that should lead to grave cardiovascular diseases this is not the case, and this type of disease was far less frequent in France. This was ascribed to the regular use of red wine which decreased to extremely low level the risks and the occurrence of cardiovascular diseases. Now not only is tannin added to the wine, but it is considered particularly beneficial to do it. It must be pointed out that it is particularly purified tannin, from which carbohydrates and other components have been eliminated. It is the antioxidant property of the polyphenolic groups of the tannin which gives to it the powerful anticardiovascular effect and positive properties. Both addition of tannin as well as addition of tannin-rich oak wood chips to wine is now completely legal in Europe.2.5. Fireproof and Insulating Foams
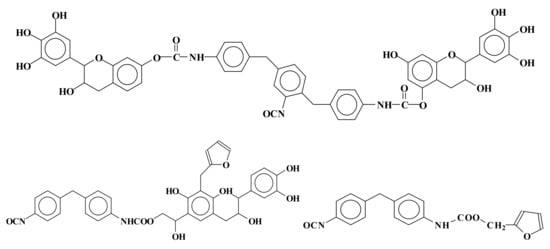
There are a number of different developments in rigid foam insulation. For imitating synthetic polyurethanes, by reaction with isocyanates, two approaches have been tried. First, foams have been developed based on the reaction of a modified tannin either by benzoylation or by oxypropylation to make it more susceptible as polyol to the reaction with polymeric isocyanates [55][56][57][58][62,63,64,65]. This first approach follows the same approach that was made with another natural polyphenol, namely lignin [59][66]. This is a traditional approach where tannin only functions as a polyol. The second approach of this type uses a very different strategy based on the reaction of a tannin with an aldehyde and the subsequent reaction of the methylol (–CH2OH) groups so formed with the isocyanate. This approach is now used for both tannin wood adhesives [18][60][25,67] and other formaldehyde adhesives such as urea-formaldehyde (UF), melamine-urea-formaldehyde (MUF) and phenol-formaldehyde (PF) [18][19][25,26]. Incidentally it is the only system that allows the formation of urethanes in an aqueous medium. Thus, according to this second approach, mixed rigid foams of phenolic–polyurethane type have been developed by reacting natural tannin/furanic mixtures with polymeric isocyanate in the proportions suitable for continuous polyurethane foam lines [61][68]. Urethane linkages formed at both the alcoholic C3 and phenolic hydroxyls. Other species in the mix were involved in urethane linkages formation: (1) glyoxal after or before its reaction with the tannin, (2) the phenolsulfonic acid catalyst, and (3) furfural. Furfural instead preferred to form methylene bridges with the flavonoids A ring than to form urethane linkages by reacting with the isocyanate. Thus, methylene and urethane bridges were formed between all the main materials in the mix. Thus, a number of mixed species bound by both types of linkages were formed. Species formed by mixed co-reaction of two, three, and four different reagents in the mix were identified. Examples of mixed species are shown in Figure 25:
Figure 25. Examples of mixed species obtained by the reaction of tannin–furfuryl alcohol–glyoxal mixes with polymeric diphenyl methane isocyanate (PMDI).
