Salivary gland regeneration is important for developing treatments for radiation-induced xerostomia, Sjögren’s syndrome, and other conditions that cause dry mouth. Culture conditions adopted from tissue engineering strategies have been used to recapitulate gland structure and function to study and regenerate the salivary glands. The purpose of this review is to highlight current trends in the field, with an emphasis on soluble factors that have been shown to improve secretory function in vitro.
- salivary gland
- tissue engineering
- cell culture
- soluble cues
- media optimization
1. Introduction
Salivary glands are organs that produce saliva, an aqueous fluid that contains enzymes, electrolytes, mucins, and other components that aid in lubricating the mouth, preliminary digestion, and antimicrobial and buffering activity to prevent oral infections [1]. There are three major pairs of salivary glands—the parotid, submandibular and sublingual—as well as numerous minor salivary glands located throughout the mouth [2]. There are also myoepithelial cells that surround the acinar units and other supportive tissues, including blood vessels, providing an exchange of nutrients and waste, and nerves, which play a key role in stimulating the glands to secrete [1]. More extensive reviews of salivary gland anatomy, physiology, and development can be found elsewhere [1][3][4][5][1,3,4,5].
The mechanisms underlying salivary gland dysfunction are not well understood and may vary greatly depending on the cause [6]. Specific to radiation-induced salivary gland dysfunction, prevention is feasible by treating with amifostine, a reactive oxygen species scavenger, and/or utilizing intensity-modulated radiation therapy (IMRT), which is a targeted approach to avoid direct radiation to the parotid glands, but these methods come with drawbacks as well [6][7][6,8].
The use of in vitro culture systems would be useful to gain more knowledge on the mechanisms leading to salivary gland dysfunction as well as enable the development of new therapies, such as cell transplantation, the discovery of new radioprotective drugs, and methods to regenerate the damaged gland. However, culture of salivary glands has historically been difficult due to the rapid loss of secretory phenotype and function in vitro.
2. Trends in In Vitro Salivary Gland Tissue Culture
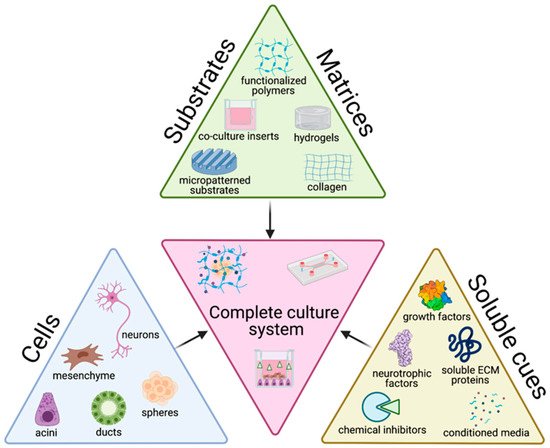
The tissue engineering paradigm is typically comprised of three main components: cells, matrix, and soluble cues. Each of these components alone, as well as the interconnectedness between these three branches have important implications on the success of culture methods. To accurately recapitulate the structure and function of native tissue, the choice of cell source and the biophysicochemical cues provided to cells are key factors in determining the success of the culture with broader impacts on experimental significance. Here, we review the cells, matrices/substrates, and soluble factors and highlight some of the combinatory approaches that have utilized multiple categories, summarized in Figure 12.
Cells are the central aspect of the tissue engineering paradigm. For the salivary glands, cell lines do not accurately represent all characteristics of normal salivary gland tissue [8][17] and many of these cell lines are contaminated with HeLa cells, including the most commonly use HSG cell line [9][10][18,19]. Additionally, pluripotent stem cell technology for the salivary gland is in its infancy [11][20]. Thus, the majority of studies evaluated use primary cells for culturing salivary gland cells (76%, Figure 23A).
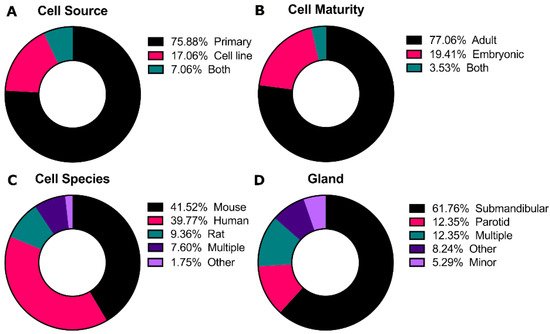
Primary cultures from adult tissue typically start with dissociation of the entire gland, containing acinar, duct, and myoepithelial cells. While some groups dissociate glands into single cells [12][13][23,26], others have highlighted the importance of maintenance of partial tissue structure to retain cell-cell contacts to promote 3D morphology and polarization [14][15][16][17][27,28,29,30]. For embryonic cultures, tissues are commonly grown as explants on a membrane with an air-liquid interface [18][19][20][31,32,33], although they can also be dissociated similar to adult tissue. In some cases, cells are selected for specific subpopulations using flow cytometry and/or selective enhancement during in vitro culture for putative stem cell markers such as CD24 and CD29, Kit, or K5 [21][22][23][24][34,35,36,37].
Co-cultures of salivary gland cells with other cell types, such as mesenchyme and nerves, have been investigated to support secretory function. For example, mouse cortical neurons were shown to self-organize around salivary gland cells similar to native tissue [25][38]. In addition, co-cultured endothelial cells were required for proper salivary gland epithelial patterning in embryonic explants [26][39]. These studies highlight the complexity of salivary gland tissue engineering and the need to consider multiple cell types.
The extracellular matrix (ECM) is a network of proteins, glycosaminoglycans, and proteoglycans that fills the intracellular space and provides structural and adhesive motifs that can influence a wide variety of cell functions such as proliferation, differentiation, and migration [27][40]. In the salivary gland epithelium, the ECM and basement membrane consist of laminin, collagen I, collagen IV, and fibronectin with binding sites for β1, β4, α5, and α6 integrins, among others [28][41]. These ECM proteins and their integrins orchestrate events during salivary gland development and homeostasis including branching morphogenesis, cleft formation, apico-basal polarization, adhesion, growth, and migration, and can influence intracellular signaling.
Of these, Matrigel®, collagen, and other polymers (mainly natural polymers) were equally as common (Figure 34B). [29][25]; hyaluronic acid-catechol that increases branching proliferation [30][42]; Matrigel®that promotes 3D structure, amylase activity, tight junction formation and transepithelial resistance (TER) [31][32][43,44]; other laminin-containing matrices that can promote morphogenesis and Aqp5 expression in combination with FGF2 [33][22]; laminin hydrogels that induced branching morphogenesis and maintained epithelial progenitors [12][23]; matrix metalloproteinase (MMP)-degradable poly(ethylene glycol) (PEG) hydrogels that were found to promote polarized expression of zona occludins (ZO-1) and sodium potassium chloride channel 1 (Nkcc1) [34][15]; and human placenta basement membrane extract or fibronectin that were shown by transmission electron microscopy (TEM) to enhance tight junction formation [32][44].
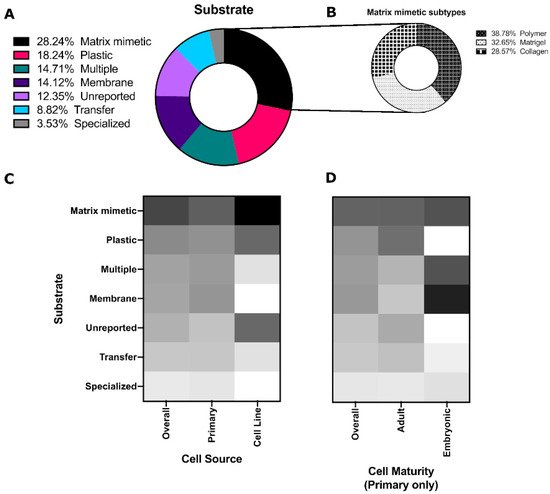
Poly(styrene) substrates (tissue culture plates, Petri dishes, etc.) were the second most common culture platform, which were predominantly used for salivary gland cell lines (Figure 34C). Membranes (Nucleopore filters, Transwells) and multiple substrates (culturing on a Matrigel®for some experiments and plastic for others) were more common for embryonic cells (Figure 34D). These polystyrene and coated substrates are standard, as many embryonic studies use explants and thus retain the structure of the developing gland without the need for extensive substrate engineering efforts.
Other substrates include: micropatterned PDMS-based craters with electrospun poly(lactic co glycolic acid) (PLGA) that were reported to increase the tight junction protein occuldin expression and expression of water channel Aqp5 [35][45]; poly(glycerol sebacate) (PGS)/PLGA core/shell nanofiber scaffolds that were reported to promote apical localization of tight junction proteins and tissue organization when combined with mesenchymal cells [36][46]; hanging drop cultures that were reported to produce microtissues mimicking tissue development [37][47]; and decellularized porcine gut matrix co-cultured with salivary gland cells and microvascular endothelial cells that were reported to promote amylase, claudin-1, and Aqp5 expression compared to 2D [38][48].
Soluble cues are typically provided to the cells through media supplementation. Use of commercial media, including HepatoSTIM, keratinocyte growth media (KGM), and bronchial epithelial growth medium (BGM), are becoming more common, although no salivary gland-specific media exists to date. There was significant overlap between different categories (ex. serum-containing media that was also supplemented with growth factors) as indicated by the Venn diagram in Figure 45A, suggesting that a variety of soluble cues are used to promote salivary gland cell growth, but specific studies contrasting individual benefits are lacking. suggesting that cells may require a different optimized medium depending on cell source and cell maturity.
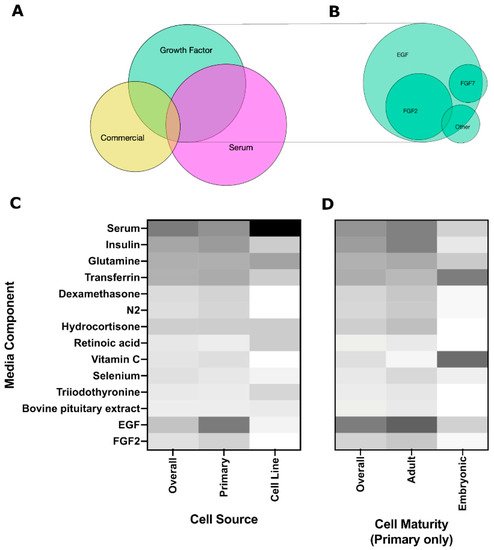
Growth factors are important in salivary gland development, with FGFs contributing to branching morphogenesis and end bud formation [39][40][58,59], while EGF contributes to cell proliferation and differentiation along the ductal lineage [41][60]. Other growth factors that are important to salivary gland development but are less commonly used include FGF7, which is reported to promote end bud formation, and FGF10, which enhances duct elongation [40][59]. Similarly, Miyajima et al., 2011 reported FGF7-induced bud expansion and FGF10-induced duct elongation, but no effect of EGF was observed on branching morphogenesis [42][24]. (IGF-1) has been shown to support growth and maintenance of the paracellular barrier function (tight junction localization, TER, dextran permeability) at levels similar to supplementation with FBS, indicating that IGF-1 could be used to replace some of the functions of FBS in a serum-free media [43][52].
Rho-kinases (ROCKs) mediate important processes such as proliferation, motility, secretion, and cell shape [44][61]. In the salivary gland specifically, ROCKs play an important role in cleft formation and basement membrane positioning in epithelial tissue polarity [45][46][62,63]. In cell culture, ROCK inhibitors have been used to prevent dissociation-induced apoptosis and preserve stem cell populations [47][48][64,65] and thus have been used to promote cell survival in vitro in many different tissue types [47][49][50][51][64,66,67,68]. Additionally, it has also been shown that ROCK activation can lead to the formation of acinar-to-ductal metaplasia under chronic pancreatitis conditions [50][67], suggesting that ROCK inhibition may prevent undesirable cell plasticity.
ROCK inhibitor Y-27632 has been shown to enhance cell growth, survival, proliferation and amylase and c-Met expression in salivary gland cultures [52][53] as well as increase sphere forming percent of CD24hi/CD29hicells [22][35]. Additionally, it may promote different cell populations —Y-27632 in serum-free media increased Kit+ cells, while K5+ cells were increased in serum-containing media [53][69], suggesting it may promote different cell phenotypes altogether. Y-27632 has been used in culturing several other tissues (pancreas, prostate, lacrimal gland) to decrease cell stress [49][50][51][66,67,68], which may account for the increased growth in ROCK inhibitor-treated cultures.
The epidermal growth factor receptor (EGFR) modulates various processes, including proliferation, differentiation, and survival [54][70], and is involved in branching morphogenesis during salivary gland development [55][71]. However, aberrant EGFR signaling can lead to cancer and other diseases [54][70] and EGFR inhibition has been used as treatment for breast, lung, and colorectal cancer [50][51][53][67,68,69].
Several EGFR inhibitors have been used in salivary gland culture, including AG1478, PD198509, PD168393, and AG1478 retained epithelial cells and AQP5 [33][22], while PD198509 did not affect K5, K19, Kit, sphere diameter or sphere count [12][23] and PD168393 inhibited carbachol (CCh)-mediated morphogenesis and proliferation of K5+ and K19+ cells [41][60]. This is supported by the role of EGFR signaling in duct morphogenesis and differentiation and proliferation of the duct lineage [56][72] and by pancreas literature in which EKI-785 was used to prevent the transition of acinar cells into duct-like clusters in vitro [57][73]. This also highlights a key consideration in the selection of an optimized media—different goals may warrant different soluble cues.
TGFβ has multiple roles in the salivary gland—it is important during morphogenesis [58][74], but it is also upregulated following stress, a major driver of fibrosis [59][75] and overexpression of TGF-β1 can lead to acinar loss [56][60][72,76]. Hence, it has been studied in salivary gland culture with different outcomes. The TGFβR inhibitor, RepSox, promoted cell growth, proliferation, expression of keratins 8, 14, and 19 and was selective for p63-expressing cells [61][77], while treatment with SB525334 increased acinar characteristics [62][50]. Since both inhibitors target TGFβR1, differences between these outcomes could be due to inhibitor potency, differences in culture conditions (tissue culture plate with commercial CnT-PR media [61][77] vs. growth factor-reduced Matrigel with DMEM/N2 media [62][50]), or difference in cell source (embryonic [61][77] vs. adult [62][50]).
Hence, adding neurotrophic factors to the culture media has been widely considered as an alternative to the complexity of co-culturing salivary gland cells with nerve cells. Results show that treatment with different nerve factors has positive effects on salivary gland cultures. , neurturin (NRTN) initiated branching and innervation [12][23], and neureglin 1 (NRG1) promoted branching and retention of acinar-like cells [63][79]. This is supported by a myriad of publications highlighting the importance of neurotrophic factors such as neurturin and glial cell-derived neurotrophic factor (GDNF) for development of the salivary gland [41][64][65][66][67][68][60,78,80,81,82,83].
Conditioned media from a variety of sources has also been shown to improve salivary gland cell culture. Wnt and R-spondin conditioned media increased long-term expansion of salivary gland stem cells in vitro, with increased population doubling and sphere-forming efficiency [69][55]. Mesenchymal stem cell ( Fibroblast-conditioned media increased amylase protein levels [70][84] and amylase expression [71][54] but was dependent on the substrate
In addition to variations in the biomaterials used for the matrix, soluble ECM proteins have also been used to provide signaling cues to the salivary gland cells as media additives. For example, fibronectin induced branching and ductal elongation [72][85], which was enhanced with FGF7. Salivary gland ECM extract (s-Ecx) promoted a compact sphere structure and increased expression of keratins (K5, K7, K14, K19) and acinar markers such as Aqp5 and Muc-1 [73][86]. Chitosan has been shown to increase spheroid size and polarization [29][25], with the greatest effect from soluble chitosan.
3. Opportunities for Future Research
Some of the most promising approaches for salivary gland cell culture involve the combination of the tissue engineering triad—cells, matrices, and soluble cues. While certain substrates have been shown to promote growth and expression of acinar markers, matrices are more relevant to the in vivo environment and versatile, providing structure, signaling through integrins, allowing for entrapment of signaling molecules and modifications with matrix motifs. In particular, laminin-based biomaterials have shown promising results [33][12][22][69][63][22,23,35,55,79]; this is supported by the prevalence of laminin in the ECM and basement membrane of the salivary gland in vivo [28][41].
Despite the promising polarization supported by matrix mimetics, secretory function remains limited. This continued challenge points to the need for incorporating combinatory approaches that optimize the matrix along with the other arms of the tissue engineering paradigm. While a number of groups have tested one or two matrix and/or media conditions, a large scale, combined media, and matrix optimization has not been done. In addition, analysis of how these factors affect a wide variety of markers, both acinar and duct, would be beneficial for more widespread adoption across the field.
Increasing complexity of models by introducing mesenchyme and neurons will enable more representative tissue mimetics for fundamental biology studies. However, simplicity may be desired in other cases for increased convenience, lower costs, and ease of data interpretation. Thus, it would be beneficial to investigate the specific function provided by supportive tissues and whether media supplementation or matrix modification can produce the same results.
