Carbon molecular sieve (CMS) membranes have been developed to replace or support energy-intensive cryogenic distillation for olefin/paraffin separation. Olefin and paraffin have similar molecular properties, but can be separated effectively by a CMS membrane with a rigid, slit-like pore structure. A variety of polymer precursors can give rise to different outcomes in terms of the structure and performance of CMS membranes.
- olefin/paraffin
- carbon molecular sieve membrane
- polymer
- precursor
- pyrolysis
1. Introduction
Extractive distillation is a common process for olefin/paraffin separation, but it is not better than traditional distillation [1][19]. For the past several decades, adsorption processes have been investigated for use in olefin/paraffin separation [2][20]. Recently, cyclic adsorption processes such as simulated moving bed (SMB), vacuum pressure swing adsorption (VPSA), and thermal swing adsorption (TSA) have been proposed.
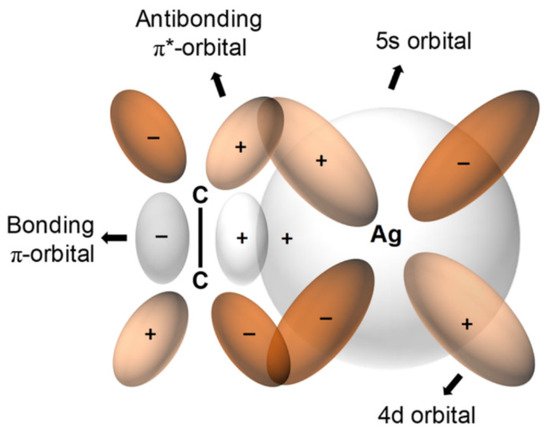
For olefin facilitated transport, transition metal cations are commonly used as carriers, thereby providing the ability to form reversible complexes of molecules with double bonds. Even though any transition metal could be used as a complexing agent, Ag+is the most frequently employed due to the lower stability of Ag+-olefin complexes (relative to those of other metal-olefin transition complexes). This relative instability allows olefin to be released more easily. Ag+-olefin complexes include the following: an overlap between the occupied π-orbital of the olefin and the empty 5s-orbital of the Ag+and between a π-bond offered by the back-donation of electrons from the occupied d-orbitals of the silver and the empty antibonding π*-orbitals of the olefin, as indicated in Figure 1 [1][19].
For olefin/paraffin separation, these separation technologies have great potential as alternatives to traditional distillation processes in terms of lower capital, operating, and energy costs. However, only a few non-distillation processes are being utilized in the petrochemical industry because these alternative processes still have inherent problems [4][26].
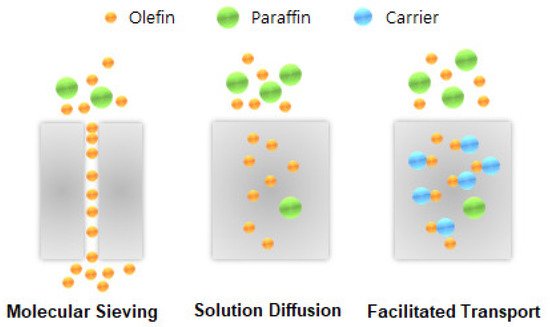
Membrane technologies offer attractive alternatives that could reduce the large capital and high operating costs of the cryogenic distillation process for olefin/paraffin separation. The membrane technologies for olefin/paraffin separation can be classified into three categories: facilitated-transport, polymeric, and inorganic membranes.
The facilitated-transport membrane is one of the most favorable and important membranes for olefin/paraffin separation [5][6][7][8][33,34,35,36]. As shown in Figure 1, metal ions are able to form reversible chemical bonds with olefins due to the π-bonding between the hybrid molecular orbitals of olefin and the metal atomic orbitals. As shown in Figure 2, this chemical complexation between transition metal ions and olefins offers high selectivity and high capacity.
The separation performance of polymeric membranes is mostly determined by polymer properties such as molecular weight, shape, polymer structure, packing, and rigidity. Among the various polymers, glassy polymers show better performance for olefin/paraffin separation, as well as for the separation of aromatic, alicyclic, and aliphatic hydrocarbons. However, it is very difficult to separate olefin from paraffin using a polymeric membrane due to the limitation of trade-off between permeability and selectivity and parasitic plasticization effects. Furthermore, polymeric membranes are not suitable for application in harsh environments [9][10][42,43].
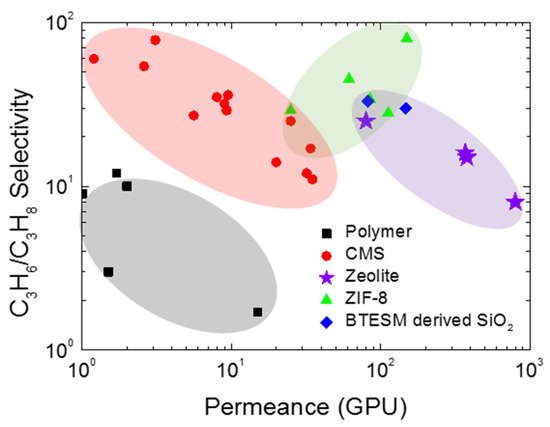
The inorganic membranes have received a great deal of attention as replacements for polymeric membranes. To surpass the upper bound trade-off for olefin/paraffin separation, recent researchers have been focusing on the development of inorganic membranes, in particular, microporous membranes such as zeolite, silica, zeolitic imidazolate frameworks (ZIFs), and CMS membranes (Figure 3) [3][11][12][13][14][25,44,45,46,47].
CMS membranes for olefin/paraffin separation have favorable performance compared with ordinary polymeric membranes [15][16][59,60]. After carbonization of the polymer precursors, the structure of CMS membranes is significantly changed to form a rigid pore wall, which gives high gas permeation and selectivity with better thermal and chemical stability [17][18][61,62]. Air products in the early 1990s developed the nanoporous carbon membranes called a selective surface flow membrane (SSFTM). CMS membrane module with the surface area of 0.5–2.0 m2was applied for the production of vehicle fuel from biogas.
2. Structure and Gas Transport Mechanism of a CMS Membrane for Olefin/Paraffin Separation
A microporous plug was prepared by compressing a high specific-surface area carbon powder to study the adsorption and diffusion of gases. From this, it was confirmed that surface flow is important for most polarizable species [19][20][68,69]. Barrer and coworkers then comprehensively investigated the gas and vapor sorption and diffusion properties [21][70] of the compacted carbon membranes, which showed excellent intrinsic performance for gas separation. Most CMS membranes are currently achieved using a process involving pyrolysis of polymeric precursors at high temperature in an O2-free condition.
In particular, CMS membranes have attracted considerable attention for olefin/paraffin separation [18][22][62,72]. The rigid pores of CMS membranes enable penetration of gas molecules with impressive permselectivity and without deformation and deterioration of the pore structure during the hydrocarbon separation. Moreover, olefin/paraffin separation often requires harsh operating conditions (polymeric membranes show abnormal behavior in this specific situation [23][24][73,74]). CMS membranes are also cost-effective and easier to process than zeolite, metal-organic frameworks (MOFs), and silica membranes [25][75].
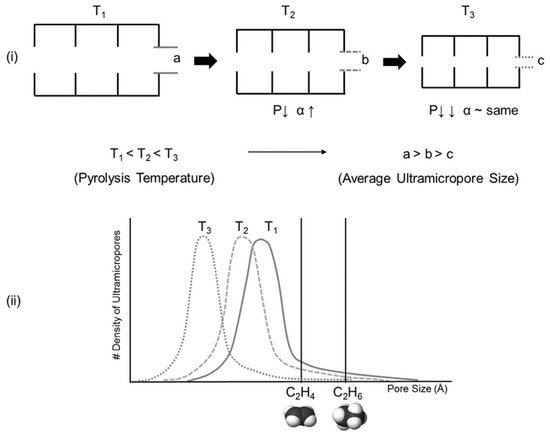
CMS membranes consists of a rigid and amorphous structure with small pore size distribution [26][27][76,77]. The pore structure of a CMS membrane has ultramicropores (0.3–0.5 nm), which allow molecular-sieving separation. However, larger micropores (0.6–2 nm) are also present and permit excellent penetration of gas due to their high sorption coefficient, which provides both high permeability and selectivity [28][29][78,79]. To allow gas diffusion by the molecular sieve mechanism, the molecules adsorbed in micropores must have more activation energy that is required to overcome repulsion from the walls of ultramicropores [30][31][64,80].
N2physisorption is the most frequently used method to characterize the pore size of CMS membrane. Besides, the pore size distribution of CMS membrane obtained from positron annihilation lifetime spectroscopy (PALS) has been often reported [32][33][34][81,82,83]. Positrons can either annihilate or trap in pores of CMS membrane. Therefore, the average positron lifetime increases with increasing pore size.
3. Polymer Precursors for CMS Membranes
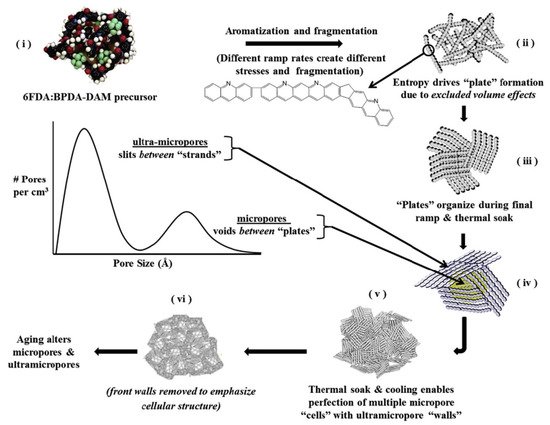
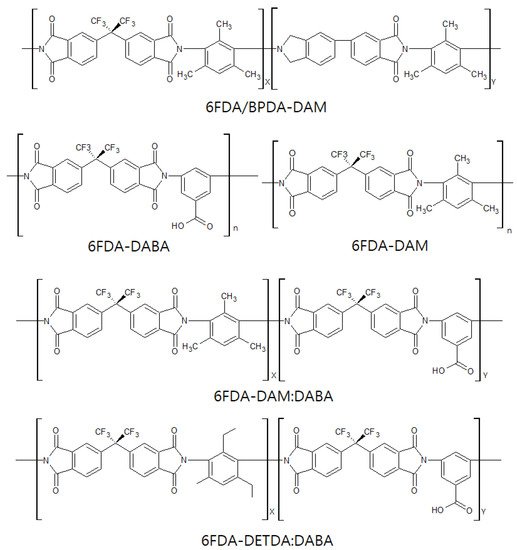
A variety of polymer precursors can produce different outcomes in terms of the structure and performance of a CMS membrane. However, it is expected that the transition process from polymer chain to amorphous or turbostratic carbon structure during pyrolysis may be similar among most of the polymer precursors because they undergo the same heat-derived processes (such as ramping, soaking, and cooling) in sequence [17][31][61,80]. There is a progression from amorphous chains of the polymer precursor to the disordered stacked plates of carbon structures during the pyrolysis process. [36][85] systematically and logically rearranged a pyrolysis protocol with 4,4′-(hexafluoroisopropylidene) diphthalic anhydride/3,3′-4,4′-biphenyl tetracarboxylic dianhydride-2,4,6-trimethyl-1,3-phenylene diamine (6FDA/BPDA-DAM) as polymer precursor, as indicated in Figure 5.
3.1. Polyimide
Polyimide is a highly aromatic polymer commonly used as a precursor of CMS membranes due to its high carbon yield, which originates from its high glass-transition temperature (Tg) and rigid structure. In addition, polyimide has the advantage of being able to tune various chemical structures, which facilitates the change of properties accordingly. Kapton and Matrimid (commercial polyimides) have frequently been used for CMS membranes, and synthesized polyimide precursors based on 6FDA and BPDA have been reported.
3.1.1. Matrimid
Matrimid is a commercial polyimide that has been widely used in the field of gas separation due to its high thermal stability and processability (Figure 6). In particular, it is a very attractive material for polymeric membranes and provides excellent permeability and selectivity. These advantages allow the CMS membrane to offer excellent performance. Thus, this representative, useful polymer precursor has been studied by many researchers.
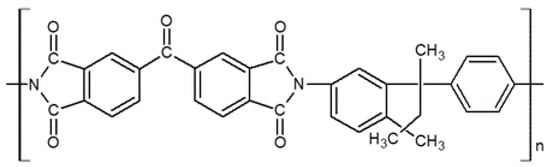
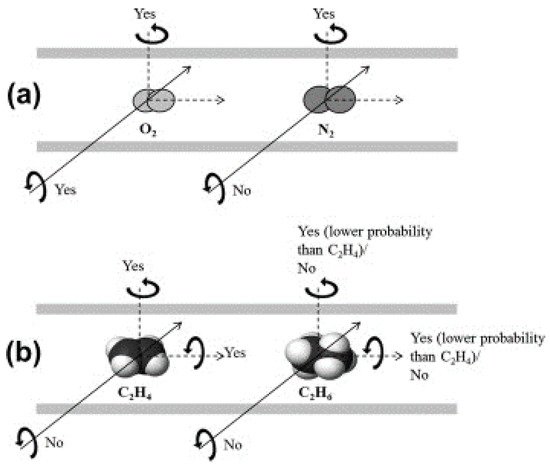
The pore structure of a Matrimid-based CMS membrane was studied using a method based on differently sized gas probes as well as XRD, positron annihilation lifetime spectroscopy (PALS), and CO2sorption. At a high pyrolysis temperature, the d-spacing of the CMS membrane (~3.8 Å) became narrower and a large number of smaller pores (<4.2 Å) were developed. Furthermore, the corrected diffusivity trends showed that a majority of the ultramicropores in the CMS membrane were in the range 2.6–3.5 Å. In particular, the more sorptive and planar C2H4can more easily penetrate the CMS membrane compared to round or bulky molecules such as CH4and C2H6 (Figure 7).
In another case, the separation performance of the CMS membrane derived from Matrimid dense film and asymmetric hollow fiber, was compared for C2H4/C2H6separation. The selectivities of both were very similar (~12), but the thickness of the carbon active layer was very different. This difference was attributed to collapse of the porous, hollow-fiber substructure due to the relatively low glass-transition temperature (Tg) of Matrimid. Nevertheless, this collapse phenomenon enabled the defective hollow fibers to transform into a highly selective CMS membrane during pyrolysis [18][62].
Commercial Matrimid material is expensive and the resulting CMS membrane is brittle and fragile. To overcome these problems, a CMS composite membrane on low-cost alumina hollow fiber was prepared. This provides high packing density, good mechanical strength, and lower material cost. It showed good separation performance: 69.2 GPU and 18 for C3H6permeance and C3H6/C3H8selectivity, respectively [37][92].
3.1.2. 6FDA-Based Polyimides
Studies on 6FDA-based CMS membranes have steadily progressed and are still reported to provide excellent performance for olefin/paraffin separation. The bulky –C(CF3)2- linkage of 6FDA-based polymers inhibits chain packing and offers a high fractional free volume, leading to high gas permeability [38][115]. In addition, it is believed that the bulky 6F group gives rise to a CMS membrane with higher gas permeability if derived from 6FDA-based polymers than if derived from Matrimid [39][116]. Furthermore, the 6FDA-based polyimides facilitate tuning of the chemical structure, which allows the polymer precursor to provide a variety of physical properties, as shown in Figure 8.
This induces a greater cumulative pore volume (4–11 Å), resulting in higher permeability and lower selectivity. The CMS membrane derived from 6FDA/BPDA-DAM polyimide, after heating to 550 °C, showed very attractive performance of 200 barrer and 100 for C3H6permeability and C3H6/C3H8selectivity, respectively [40][88]. Furthermore, Xu et al. reported that a CMS membrane derived from 6FDA/BPDA-DAM hollow fiber maintained a better asymmetric structure during pyrolysis, while a substructure collapse with related loss of permeance was found in a Matrimid-based CMS membrane. This is attributed to a higher Tg(424 °C) and greater rigidity of the 6FDA/BPDA-DAM polyimide, leading to a thin active carbon layer and higher permeance [41][90].
Pre-crosslinking of a DABA-containing 6FDA-based polyimide at a temperature below Tghas been done [42][43][117,118]. This improved the chemical and physical stability of the polymer material and resulted in an increase of the gas permeability of the polymeric membrane. (6FDA-DABA) polyimide was utilized to prepare CMS membranes (Figure 9) [44][93]. The pre-crosslinked CMS membrane possessed higher graphitic carbon content, which induced π-π interaction between the C3H6and graphitic carbons, resulting in remarkably increased C3H6/C3H8selectivity.
Fu et al. investigated the effect of O2doping and pre-crosslinking of 4,4′-(hexafluoroisopropylidene) diphthalic anhydride/2,5-diethyl-6-methyl-1,3-diaminobenzene-3,5-diaminobenzoic acid (6FDA/DETDA-DABA)-based CMS membranes on their performance for C3H6/C3H8separation [27][77]. The use of O2doping as a fine-tuning method gave rise to a narrower ultramicropore structure in the CMS membrane and enhanced C3H6/C3H8selectivity by more than 50. With pre-crosslinking, limiting the movement of polymer chains creates microvoids and packing disruptions, as shown in Figure 10.
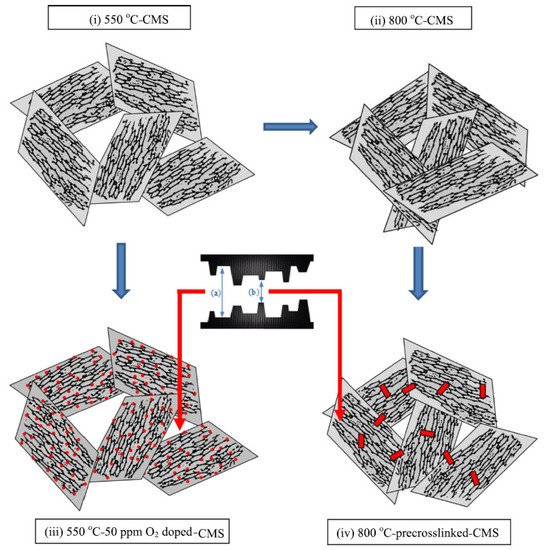
This structure might be maintained after the subsequent pyrolysis; therefore, the available sorption sites could be increased and the permeability significantly enhanced with only a slight loss of selectivity.
CMS membranes derived from 6FDA-based polyimide have also been developed on inorganic supports due to its brittleness and handling issues in practical applications of non-supported CMS membranes. Furthermore, the collapse of the porous substructure of asymmetric CMS hollow fibers was often reported, which collapse causes reduction of gas permeance. prepared 6FDA-based CMS composite membranes on an alumina disc with intermediate layer [9][42]. Moreover, the effects of feed pressure, gas fraction, operating temperature, and carbon layer thickness on the C3H6/C3H8separation performance were thoroughly studied [25][45][75,96].
Therefore, a CMS membrane that exceeds the upper bound performance and has excellent stability is promising either to replace or to support, the energy-intensive distillation process. However, it is difficult to completely replace the conventional distillation process with membrane-only processes due to limitations on separation performance. L. Xu et al. suggested a new hybrid process with an olefins-selective membrane unit and two distillation columns, or a series of olefin-selective membrane units and a distillation column [41][90]. Furthermore, a techno-economic analysis of the hybrid process (membrane followed by distillation) for C3H6/C3H8separation resulted in a lower total cost of 13.1% compared to the conventional distillation process [46][94].
3.1.3. Other Polyimides
BPDA could easily be imidized with diamines at high temperature, and the derived CMS membrane showed excellent separation performance after an additional oxidation process. Optimization of CMS membrane derived from 3,3′-4,4′-biphenyl tetracarboxylic dianhydride-4,4′-oxydianiline/diaminotoluene (BPDA-ODA/DAT) could be achieved by oxidation in air at 400 °C while the gas permeance of a CMS membrane without oxidation post-treatment was much lower [47][98]. The change of pore structure of a CMS membrane derived from BPDA-ODA according to pyrolysis temperature was also studied. The slit-like pores were gradually decreased with increasing pyrolysis temperature, resulting in the reduction of gas permeance.
In another study, CMS membranes derived from 1,4,5,8-naphthalene tetracarboxylic dianhydride (NTDA)-based sulfonated polyimides were prepared. The NTDA-based polyimide was synthesized by condensation polymerization and thermal imidization using chemicals with sulfonic groups such as benzidine-2,2′-disulfonic acid (BDSA), 4,4′-diaminodiphenylether-3,3′-disulfonic acid (ODADS), 9,9′-bis(4-aminophenyl)fluorene (BAPF), and 2,2-bis [4-(4-aminopheoxy)phenyl]hexafluoropropane disulfonic acid (BAHFDS). The large amount of microvoids caused by the template-like effect of sulfonic groups gave rise to higher gas permeability. Therefore, higher content of sulfonic groups led to increase in the gas permeability [48][39].
Kapton is another representative commercial polyimide precursor of CMS membranes, besides Matrimid. A CMS membrane made from Kapton polyimide precursor could be prepared without modification because it has homogeneous fine pores without defects such as cracks or large pores [49][119]. Suda et al. carried out mild activation of a Kapton-based CMS membrane at 400 °C under Ar gas to enlarge the pore size, and obtained the increased pore size distribution of 3.7–3.9 Å The modified CMS membrane showed high C2H4and C3H6permeance of 55.58 and 11.85 barrer and C2H4/C2H6and C3H6/C3H8selectivity of 5.82 and 25.27, respectively [50][97].
Recently, CMS membrane derived from hydroxyl polyimide (HPI-HD5) as a representative TR-able polyimide was introduced, but this is not for olefin/paraffin separation. The HPI-HD5 was thermally treated for thermally rearranged (TR) conversion and carbonized in IR furnace. The carbonization of polymer precursor in IR furnace containing oxygen gave rise to less processing time and energy requirement. This can improve the productivity by hundreds of times compared to that of conventional electrical furnace [51][120].
3.2. Phenolic Resin
Phenolic resin is a very attractive material for CMS membranes due to its thermosetting, high heat resistance, and carbon yielding properties [52][121]. Furthermore, it is a very inexpensive polymer. For these reasons, it is applied in a variety fields. This attractive polymer is being used as a precursor to create CMS membranes for gas separation, but in some studies, these membranes have been used for olefin/paraffin separation.
A thermal curing process is typically part of the preparation of CMS membranes derived from phenolic resin; this provides high thermal resistance and maintains the integrity of the membrane during pyrolysis. The former creates the O2bridge between aromatic molecules, which results in more open porosity and in better performance for olefin/paraffin separation. The latter enhances the surface diffusion of condensable hydrocarbon by adsorption in micropores instead of via the molecular sieve mechanism [24][53][54][74,102,107]. These researchers also systematically studied the effects of pyrolysis temperature, heating rate, soaking time, and atmosphere on the separation performance of CMS membranes [55][106].
3.3. Polymer of Intrinsic Microporosity (PIM)
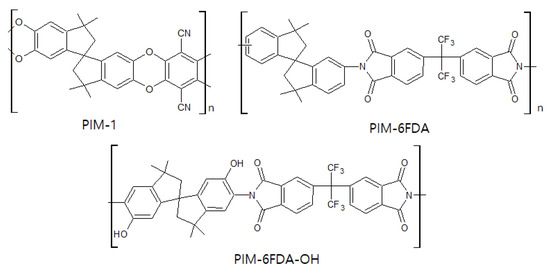
PIM is a relatively new, state of the art material for CMS membranes, as well as a polymer membrane used for gas separation. As shown in Figure 112, this polymer has a rigid backbone that induces inefficient packing of chains in the solid state, which condition provides high surface area, high free volume, and undetectable Tg[56][57][108,110]. These properties enable it to provide excellent separation performance with good flexibility and processability. A CMS membrane derived from PIM-6FDA-OH showed higher C2H4permeability (10 barrer) and C2H4/C2H6selectivity of 17.5 compared to that of the PIM-1-based CMS membrane [58][111].
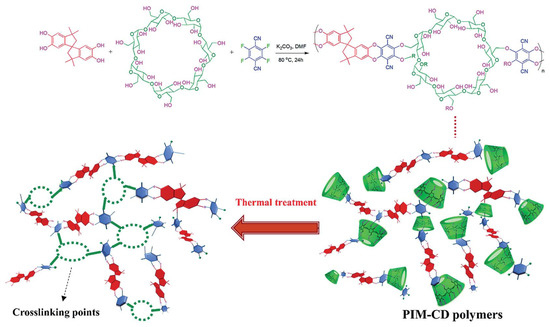
With increase in the thermal treatment temperature, the thermally liable CDs were decomposed. The 3-D network provided selective, narrow gates without loss of gas diffusion (Figure 123). However, there was concern about severe packing and shrinkage of micropores and ultrafine micropores by excessive crosslinking and carbonization at high temperature because these could decrease the gas permeability. The CMS membrane derived from PIM-CD at 400 °C showed very high C3H6permeability (2093 barrer) with low C3H6/C3H8selectivity (5.19).
3.4. Other Polymers
3.4. Other Polymers
Many other polymer precursors, such as polyacrylonitrile (PAN), polyfurfuryl alcohol (PFA), polyetherimide (PEI), poly(2,6-dimethyl-1,4-phenylene oxide) However, among them, only a few precursors were reported useful for olefin/paraffin separation due to their complex synthetic processes, relatively low free volume, poor separation performance, and poor processability. The semi-IPN matrix provided stronger thermal stability and higher carbon yield than pure PAEK did. In addition, the CMS membrane derived from PAEK-Azide (80:20) showed good separation performance of 48 barrer and 44 for C3H6permeability and C3H6/C3H8selectivity, respectively.
prepared a CMS membrane derived from a polyester-resin precursor prepared by crosslinking unsaturated polyester and styrene with a 3-D network [26][76]. The CMS membrane, which had a very thin carbon layer (125 nm) on an alumina tubular support, was applied for C4H8/C4H10separation. showed very high C4H8permeance (574 GPU). However, low C4H8/C4H10selectivity (1.93) was observed because this membrane was prepared for H2/hydrocarbon separation.
3.5. Inorganic-Containing Polymers
To improve their separation performance and physical properties, inorganic nanoparticles, MOFs, and carbon-based nanomaterials have been introduced into the matrix of membranes. Incorporating such inorganic materials into CMS membranes has been also employed. Teixeira et al. reported a CMS membrane derived from phenolic resin precursor loaded with boehmite nanoparticles. The boehmite was transformed to alumina via dihydroxylation at high temperature, which process provided a high carbon yield.
For a carbon-silica membrane, a polyimide-silica precursor was prepared by mixing tetraethyl orthosilicate (TEOS, the silica precursor) and diethoxydimethylsilane (DEDMS, the silica-network modifier) in poly(acrylic acid) The well-dispersed spherical silica particles and the spaces between the carbon matrix and silica particles facilitated gas permeation. At the same time, a carbon matrix offered a selective domain, which provided good separation performance compared with other carbon membranes. The resultant gas permeability and selectivity were 398 barrer and 5.3 for C2H4/C2H6, and 375 barrer and 25.0 for C3H6/C3H8separation [60][114].
Various boron compounds with different molecular sizes were incorporated into a hydrolyzed PIM-1 as the polymer precursor [56][108]. The pore size of the boron-embedded CMS membranes increased, and the number of pores increased, due to the large size of the boron compound. This CMS membrane exhibited higher gas diffusivity and greater diffusion selectivity: C2H4permeability of 13.7 barrer and C2H4/C2H6selectivity of 9.7 in mixed gases.
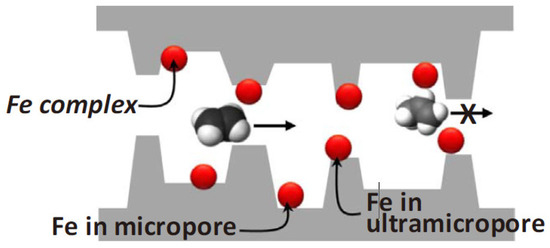
They employed 1.1–3.2 wt% transition metal ions incorporated into the 6FDA-DAM/DABA polymer precursor and pyrolyzed under various conditions. The most suitable CMS membrane was obtained at the pyrolysis temperature of 550 °C using a rapid ramp rate due to the interaction between olefins and active Fe2+cations. The slow-pyrolysis protocol can induce oxidation of Fe, which reduces the sorption selectivity. Furthermore, the Fe complex provided greater diffusion selectivity by blocking less selective ultramicropores, as shown in Figure 136, resulting in C2H4permeability of 100 barrer and C2H4/C2H6selectivity of 8.53.
4. Aging and Stability Issues of CMS Membranes
CMS membranes are undergoing a challenge of low mechanical strength due to brittle property after carbonization. This may be mitigated by optimizing the polymer precursor and preparation process [62][63][64][128,129,130]. However, enhancing the mechanical strength by these methods is limited because it is related to the performance of the CMS membrane.
The membrane configuration has significant impact on the mechanical stability of CMS membrane. The hollow fiber morphology which has relatively higher mechanical strength compared with flat sheet facilitates the modulation of CMS membranes, achieving the defect-free carbon layer as well as offering the high packing density [65][131]. In particular, supported CMS membrane is ideal to provide commercially-viable mechanical strength, which is favorable for operating at high temperature and high pressure. Therefore, composite CMS membrane can provide both high performances and mechanical stability.
On the other hand, a pre-crosslinking of polymer precursor can increase the flexibility of the CMS membrane. A crosslinked polymer generally becomes more brittle due to increase in the rigidity of the polymer chains. However, the CMS membrane undergoes the procedures of decomposition, aromatization, and fragmentation during pyrolysis, leading to rigid graphene-like layers with high fragility and brittleness [66][132]. This characteristic might be alleviated by the crosslinking between polymer chains, resulting in a more flexible CMS membrane.
Chemical aging in a CMS membrane is induced by adsorption or interaction with external species such as organic contaminants, O2(or air), and humidity, as illustrated in Figure 147. This causes severe degradation of gas permeation due to plugging of the pore structure [67][91].
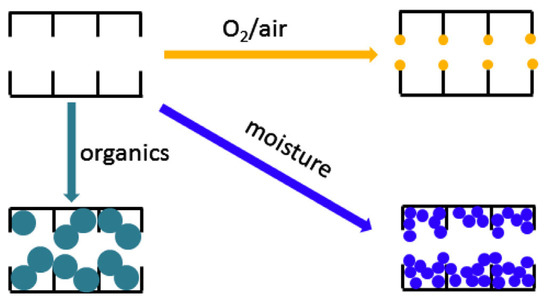
For chemical aging by organic contaminants, Jones et al. exposed CMS membranes to hexane, vacuum pump oil, phenol, and toluene, resulting in reduction of gas permeance due to the hydrophobic property of carbon [68][134]. However, the chemically aged pore structure of a CMS membrane could be regenerated simply by exposure to C3H6.
Menendez et al. investigated chemical aging by exposure to air, N2, and C3H6of a phenolic resin-based CMS membrane. In particular, when the CMS membrane was exposed to O2, the permeance was significantly decreased, which may be attributed to reactive edge sites in the carbon structure for O2chemisorption [69][103]. Moreover, the water was strongly adsorbed to the CMS membrane in high humidity, leading to the formation of water clusters on hydrophobic carbon pores. The adsorption of water significantly decreased gas permeance by reducing the available pore volume and pore size, as with aging by organic components.
Despite many attempted solutions aimed at recovering from the chemical aging of CMS membranes, this unwanted phenomenon is still a critical issue in their commercialization. A fundamental solution to chemical aging must be developed in further work.
Recently, an unexpected form of permeance loss was observed while CMS membranes were stored under vacuum or in O2-free dry conditions. This observation was attributed to physical aging in 2014 by Xu et al. Moreover, physical aging was observed in continuous gas permeation processes under O2-free conditions. This team hypothesized that CMS membranes undergo physical rearrangements to reach thermodynamic equilibrium due to initial imperfections of the graphene-like layers, as indicated in Figure 158.
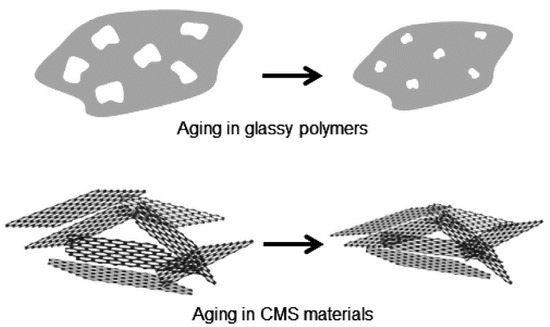
Some efforts aimed at avoiding permeance loss by CMS membranes due to physical aging have been reported. There are other studies that, even though they were not about olefin/paraffin separation, did involve efforts to prevent physical aging. This technique prevented physical aging to some extent, under a continuous active feed of 50 psia. In another case, a polydimethylsiloxane (PDMS) coating on the surface of a CMS membrane was employed to delay physical aging, even though this could not completely prevent the physical aging.
To apply CMS membranes at industrial scale, it is crucial to overcome this aging phenomenon to guarantee long-term operation. Based on the literature on this topic, it is clear that the shrinkage or densification of graphene-like layers as steps toward achieving a state of thermodynamic equilibrium must be prevented. Therefore, ways to modify or apply pre-/post-treatment of CMS membranes will be key technologies.
