Diabetic cardiomyopathy (DCM) is a constellation of symptoms consisting of ventricular dysfunction and cardiomyocyte disarray in the presence of diabetes. The exact cause of this type of cardiomyopathy is still unknown; however, several processes involving the mitochondria, such as lipid and glucose metabolism, reactive oxygen species (ROS) production, apoptosis, autophagy and mitochondrial biogenesis have been implicated. In addition, polyphenols have been shown to improve the progression of diabetes. In this review, we discuss some of the mechanisms by which polyphenols, particularly resveratrol, play a role in slowing the progression of DCM. Our dissection of these molecular players aims to provide potential therapeutic targets for the treatment of DCM.
- resveratrol
- diabetic cardiomyopathy
- nutraceuticals
- polyphenols
- mitochondria
1. Introduction
Diabetic cardiomyopathy (DCM) is a multifactorial phenotype consisting of ventricular dysfunction in the absence of other cardiac risk factors such as coronary artery disease, hypertension and significant valvular disease in the presence of diabetes [
1]. This dysfunction may progress into outright heart failure with preserved or reduced ejection fraction [1], and it is a major cause of morbidity and mortality in the diabetic population. The definitive cause for DCM is still unclear, yet many contributing factors have been reported. These include hyperglycemia, impaired lipid oxidation, lipid accumulation in the cardiomyocytes, deposition of advanced glycated end products and endothelial dysfunction [2]. Increased apoptosis and impaired autophagy resulting from these disturbances have been also implicated in the cardiac remodeling seen in DCM [3]. Autophagy is the process by which damaged proteins and disabled organelles including mitochondria are degraded and recycled within lysosomes [4]. The double membrane bound entity that the lysosome forms with the degeneration products is known as the autophagosome. Autophagy is a normal and necessary process within the cell, but under stressful conditions, it can become dysregulated and leads to apoptosis [
4]. This process can occur due to the extensive crosstalk between apoptotic and autophagic pathways, in which the mitochondria play a central role [5].
Additionally, mitochondrial biogenesis also contributes to the pathogenesis of DCM [6]. Mitochondrial biogenesis is the process by which the cell regulates gene expression of nuclear and mitochondrial genes in order to enhance ATP production. This process is usually upregulated in times of cellular stress, such as in the diabetic state [6]. Given that the mitochondria regulate many bioenergetic processes, such as lipid and glucose metabolism [7], reactive oxygen species (ROS) production and regulation [8], apoptosis and autophagy [9], its dysregulation is unsurprisingly causal for DCM.
Polyphenols are secondary plant metabolites that have been shown to improve diabetes progression and complications through various mechanisms [10], such as improving levels of oxidative stress [11] as well as enhancing insulin sensitivity [12] and lipid metabolism [13]. Polyphenols are nutraceuticals which modulate apoptosis and oxidative stress in various cell types, albeit in a cell context-dependent manner [14][15][16][17][18]. Resveratrol is a type of polyphenol found in various plants, particularly grapes and berries, and it plays important roles in human physiology and pathophysiology [19][20][21]. Some of the important mechanisms by which resveratrol elicits its effects involves the secondary messengers, cAMP and cGMP [22][23][24]. These messengers are well known to evoke pleiotropic functions, prime of which is their cardiovascular effect.
2. Effect of Resveratrol on the Mitochondrial ROS Generation and Apoptosis Pathways
In a diabetic state, there is strong evidence of increased levels of ROS production from the mitochondria and a subsequent increase in cellular apoptosis [3]. In various studies, resveratrol has been shown to modulate the amount of ROS generation under diabetic conditions through a number of pathways, thereby attenuating the acuity of resultant apoptosis. Mitochondrial uncoupling protein 2 (UCP2), a proton transporter located in the inner mitochondrial membrane, is a key mediator in the apoptotic pathway and has the capability of ameliorating ROS generation by dissipating the mitochondrial proton gradient and mitochondrial membrane potential [25].
In a diabetic state, there is strong evidence of increased levels of ROS production from the mitochondria and a subsequent increase in cellular apoptosis [3]. In various studies, resveratrol has been shown to modulate the amount of ROS generation under diabetic conditions through a number of pathways, thereby attenuating the acuity of resultant apoptosis. Mitochondrial uncoupling protein 2 (UCP2), a proton transporter located in the inner mitochondrial membrane, is a key mediator in the apoptotic pathway and has the capability of ameliorating ROS generation by dissipating the mitochondrial proton gradient and mitochondrial membrane potential [39].Increased ROS has been shown to increase the likelihood of arrhythmias [26], particularly in structurally damaged hearts with left ventricular hypertrophy such as those seen in diabetic cardiomyopathy [1]. UCP2 dysregulation leads to the alteration of mitochondrial membrane potential in this case, thus contributing to the development of arrhythmias, which may evolve to ventricular fibrillation [26]. Mitochondrial oxidative stress can also precipitate atrial fibrillation [27]. Furthermore, mitochondrial dysfunction has been reported to associate with arrhythmogenic substrates in diabetes [28]. In a recent study, it has been shown that resveratrol ameliorates cardiac dysfunction in diabetic mice via the UCP2 pathway [29]. In this regard, incubation of rat cardiomyocytes with high glucose (HG) and resveratrol, significantly increases UCP2 expression, whereas siRNA knockdown of UCP2 expression inhibits the protective effect of resveratrol. In addition, siRNA knockdown of UCP2 increases the apoptotic rate of HG/resveratrol-treated cardiomyocytes, indicating that resveratrol administration also protects against apoptosis via the UCP2 pathway [29] (
Increased ROS has been shown to increase the likelihood of arrhythmias [40], particularly in structurally damaged hearts with left ventricular hypertrophy such as those seen in diabetic cardiomyopathy [1]. UCP2 dysregulation leads to the alteration of mitochondrial membrane potential in this case, thus contributing to the development of arrhythmias, which may evolve to ventricular fibrillation [40]. Mitochondrial oxidative stress can also precipitate atrial fibrillation [41]. Furthermore, mitochondrial dysfunction has been reported to associate with arrhythmogenic substrates in diabetes [42]. In a recent study, it has been shown that resveratrol ameliorates cardiac dysfunction in diabetic mice via the UCP2 pathway [43]. In this regard, incubation of rat cardiomyocytes with high glucose (HG) and resveratrol, significantly increases UCP2 expression, whereas siRNA knockdown of UCP2 expression inhibits the protective effect of resveratrol. In addition, siRNA knockdown of UCP2 increases the apoptotic rate of HG/resveratrol-treated cardiomyocytes, indicating that resveratrol administration also protects against apoptosis via the UCP2 pathway [43] (Figure 1). Interestingly, the antioxidant effect of resveratrol on diabetes-induced oxidative stress has been shown to be mediated by downregulation of UCP2 expression [30]. This could be plausibly explained in the light of the fact that UCP2 is activated by ROS as a feedback mechanism [31]. With that said, it is enticing to speculate that resveratrol appears to restore the normal level of UCP2 by countering mitochondrial insult, ROS production, as it induces the antioxidant gene expression of manganese superoxide dismutase [32]. These findings also position resveratrol as a potential therapeutic agent for arrhythmias related to diabetic cardiomyopathy and other structural heart conditions.
). Interestingly, the antioxidant effect of resveratrol on diabetes-induced oxidative stress has been shown to be mediated by downregulation of UCP2 expression [44]. This could be plausibly explained in the light of the fact that UCP2 is activated by ROS as a feedback mechanism [45]. With that said, it is enticing to speculate that resveratrol appears to restore the normal level of UCP2 by countering mitochondrial insult, ROS production, as it induces the antioxidant gene expression of manganese superoxide dismutase [46]. These findings also position resveratrol as a potential therapeutic agent for arrhythmias related to diabetic cardiomyopathy and other structural heart conditions.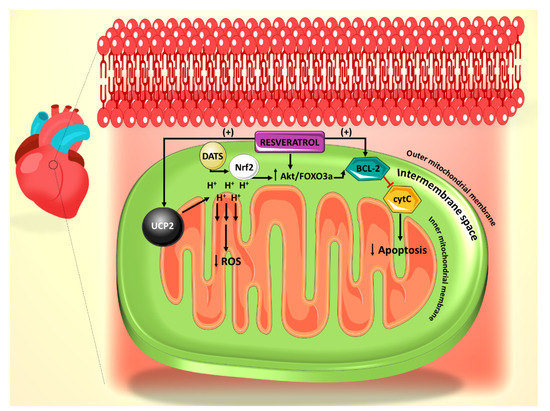
Figure 1. Summary of the effect of resveratrol on the mitochondrial reactive oxygen species (ROS) generation and apoptosis pathways. Resveratrol action is primarily mediated through UCP2 and Bcl-2. Diallyl trisulfide (DATS) is another polyphenol derived from garlic, which mediates its anti-apoptotic effect through Nrf2.
Summary of the effect of resveratrol on the mitochondrial reactive oxygen species (ROS) generation and apoptosis pathways. Resveratrol action is primarily mediated through UCP2 and Bcl-2. Diallyl trisulfide (DATS) is another polyphenol derived from garlic, which mediates its anti-apoptotic effect through Nrf2.The mitochondrial permeability transition pore (mPTP)-cytochrome c pathway is also involved in apoptosis, with cytochrome c release from the mitochondrial inner membrane being a marker for this cell death [33]. It has been shown that mPTP-cytochrome c pathway is also involved in the resveratrol-induced anti-apoptotic effect, as resveratrol treatment leads to preserved cytochrome c levels in cardiomyocytes [29]. In addition, resveratrol treatment increased the expression of B-cell lymphoma 2 (Bcl-2), a well-established anti-apoptotic factor (
The mitochondrial permeability transition pore (mPTP)-cytochrome c pathway is also involved in apoptosis, with cytochrome c release from the mitochondrial inner membrane being a marker for this cell death [47]. It has been shown that mPTP-cytochrome c pathway is also involved in the resveratrol-induced anti-apoptotic effect, as resveratrol treatment leads to preserved cytochrome c levels in cardiomyocytes [43]. In addition, resveratrol treatment increased the expression of B-cell lymphoma 2 (Bcl-2), a well-established anti-apoptotic factor (Figure 1).
).3. Effect of Resveratrol on the SIRT1-Dependent Mitochondrial Biogenesis Pathway
Resveratrol also plays a role in modulating mitochondrial biogenesis pathways, impairment of which has been implicated in the pathogenesis of diabetic cardiomyopathy. Sirtuin 1 (SIRT1), nuclear factor kappa B (NF-κB) p65 and peroxisome proliferator-activated receptor delta (PPAR-δ) have been found to be important molecular targets of resveratrol-induced modulation of mitochondrial biogenesis pathways.
Resveratrol also plays a role in modulating mitochondrial biogenesis pathways, impairment of which has been implicated in the pathogenesis of diabetic cardiomyopathy. Sirtuin 1 (SIRT1), nuclear factor kappa B (NF-κB) p65 and peroxisome proliferator-activated receptor delta (PPAR-δ) have been found to be important molecular targets of resveratrol-induced modulation of mitochondrial biogenesis pathways.SIRT1 is a NAD
SIRT1 is a NAD+-dependent deacetylase which plays a role in various mitochondrial pathways [34]. In particular, it causes the deacetylation of peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1α, a transcription coactivator which plays a central role in the regulation of mitochondrial biogenesis [35]. In a recent study, resveratrol has been shown to decrease hyperglycemia-induced injury to cardiomyocytes by increasing mitochondrial biogenesis via the SIRT1-PGC-1α pathway [30]. Conversely, when SIRT1 is inhibited by sirtinol or silenced with siRNA, the protective effects of resveratrol on the mitochondrial biogenesis are abolished [30]. Additionally, SIRT1 pathway activation seems to underlie resveratrol’s protective effect on hyperglycemia-induced hypertrophy of cardiomyocytes as evidenced by the attenuated expression of the pro-hypertrophic markers atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and β myosin heavy chain (β-MHC) [36]. In view of that, resveratrol presents itself as an important potential therapy in conjunction with other cardioprotective treatments.
-dependent deacetylase which plays a role in various mitochondrial pathways [59]. In particular, it causes the deacetylation of peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1α, a transcription coactivator which plays a central role in the regulation of mitochondrial biogenesis [60]. In a recent study, resveratrol has been shown to decrease hyperglycemia-induced injury to cardiomyocytes by increasing mitochondrial biogenesis via the SIRT1-PGC-1α pathway [44]. Conversely, when SIRT1 is inhibited by sirtinol or silenced with siRNA, the protective effects of resveratrol on the mitochondrial biogenesis are abolished [44]. Additionally, SIRT1 pathway activation seems to underlie resveratrol’s protective effect on hyperglycemia-induced hypertrophy of cardiomyocytes as evidenced by the attenuated expression of the pro-hypertrophic markers atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and β myosin heavy chain (β-MHC) [61]. In view of that, resveratrol presents itself as an important potential therapy in conjunction with other cardioprotective treatments.4. Role of Resveratrol on Mitochondrial Lipid Oxidation
Impaired lipid metabolism is a hallmark of the diabetic phenotype, and this dysregulation has been especially implicated in the development of DCM [37]. The heart uses fatty acids as its primary source of energy over glucose. However, in a diabetic state, the proportion of fatty acids to glucose usage is even higher due to insulin resistance and decreased glucose uptake [38]. The increased fatty acid uptake exceeds oxidation rates in the heart, thereby resulting in augmented accumulation of reactive lipids in cardiomyocytes and increased ROS generation due to β-oxidation of accumulated lipids [37]. It has been suggested that the surplus ROS production results in an impaired mitochondrial fatty acid oxidation as a feedback mechanism to prevent excessive reactive lipid accumulation within mitochondria [39]. Further, the mitochondrial lipid intermediates, ceramides, are known to promote apoptosis of cardiomyocytes [40]. These metabolic alterations contribute to an increased stress on the diabetic heart and ultimately lead to cardiac remodeling and dysfunction [37].
Impaired lipid metabolism is a hallmark of the diabetic phenotype, and this dysregulation has been especially implicated in the development of DCM [70]. The heart uses fatty acids as its primary source of energy over glucose. However, in a diabetic state, the proportion of fatty acids to glucose usage is even higher due to insulin resistance and decreased glucose uptake [71]. The increased fatty acid uptake exceeds oxidation rates in the heart, thereby resulting in augmented accumulation of reactive lipids in cardiomyocytes and increased ROS generation due to β-oxidation of accumulated lipids [70]. It has been suggested that the surplus ROS production results in an impaired mitochondrial fatty acid oxidation as a feedback mechanism to prevent excessive reactive lipid accumulation within mitochondria [72]. Further, the mitochondrial lipid intermediates, ceramides, are known to promote apoptosis of cardiomyocytes [73]. These metabolic alterations contribute to an increased stress on the diabetic heart and ultimately lead to cardiac remodeling and dysfunction [70].Indeed, a dysfunctional mitochondrial oxidation of palmitoyl CoA, a major lipid source available in vivo, has been observed before overt diabetes and cardiac dysfunction manifested in Zucker diabetic fatty rat models. Furthermore, accumulation of reactive lipids, increased mitochondrial ROS emission rates and elevated levels of ceramide have been also elicited in these animal models [39]. Supplementation with resveratrol improves the impaired mitochondrial respiratory sensitivity to palmitoyl CoA, ameliorates the abnormally high levels of ROS and normalizes ceramides levels and reactive lipid accumulation. Taken together, these findings point towards an important role for resveratrol as a potential regulator of the lipid dysfunction seen in DCM and present an interesting possibility to the use of polyphenol in prediabetic patients for potential protection against impaired lipid oxidation.
Indeed, a dysfunctional mitochondrial oxidation of palmitoyl CoA, a major lipid source available in vivo, has been observed before overt diabetes and cardiac dysfunction manifested in Zucker diabetic fatty rat models. Furthermore, accumulation of reactive lipids, increased mitochondrial ROS emission rates and elevated levels of ceramide have been also elicited in these animal models [72]. Supplementation with resveratrol improves the impaired mitochondrial respiratory sensitivity to palmitoyl CoA, ameliorates the abnormally high levels of ROS and normalizes ceramides levels and reactive lipid accumulation. Taken together, these findings point towards an important role for resveratrol as a potential regulator of the lipid dysfunction seen in DCM and present an interesting possibility to the use of polyphenol in prediabetic patients for potential protection against impaired lipid oxidation.5. Role of Polyphenols in Autophagy–Apoptosis Interactions and Their Role in Diabetic Cardiomyopathy
The role of autophagy and apoptosis in cellular stress, particularly that resulting from diabetes and cardiovascular dysfunction, has been well elucidated [3][41]. In general, it has been shown that autophagy in cardiomyocytes is suppressed in the diabetic state [42], whereas apoptosis is highly active [3]. These two cellular processes, although seem discrete, are interrelated with each other. Autophagy dysregulation leads to accumulation of damaged mitochondria and increased mitochondrial membrane permeabilization, resulting in the release of pro-apoptotic proteins, such as cytochrome c, which can activate caspase-mediated apoptosis [43]. Moreover, they are regulated by some common signaling pathways, thus exhibiting a significant amount of crosstalk [44].
The role of autophagy and apoptosis in cellular stress, particularly that resulting from diabetes and cardiovascular dysfunction, has been well elucidated [3,78]. In general, it has been shown that autophagy in cardiomyocytes is suppressed in the diabetic state [79], whereas apoptosis is highly active [3]. These two cellular processes, although seem discrete, are interrelated with each other. Autophagy dysregulation leads to accumulation of damaged mitochondria and increased mitochondrial membrane permeabilization, resulting in the release of pro-apoptotic proteins, such as cytochrome c, which can activate caspase-mediated apoptosis [80]. Moreover, they are regulated by some common signaling pathways, thus exhibiting a significant amount of crosstalk [81].A recent study showed that rat cardiomyocytes cultured in palmitate (PA) and high D-glucose (HG) medium, mimicking that of a diabetic heart, exhibit suppressed autophagy, made evident by the decreased lipidated protein microtubule-associated protein 1A/1B-light chain 3 2 (LC3-II) and the elevated p62 levels, which are believed to be involved in autophagosome membrane expansion and autophagic flux, respectively [45]. Treatment of cardiomyocytes exposed to HG/PA with resveratrol results in p62 downregulation and LC3-II upregulation, indicating increased autophagic flux and enhanced autophagy [45]. Resveratrol treatment also decreases the amount of apoptosis induced by the HG/PA conditions. These two actions of resveratrol on the cells have been shown to be linked, as the autophagy inhibitor 3-methylademine (3-MA) diminishes RES’s effect on both processes [45]. Moreover, it has been shown that resveratrol exerts its effect on these processes via increasing phosphorylation of AMPK, thereby inhibiting the mTOR pathway and consequently increasing levels of LC3-II (
A recent study showed that rat cardiomyocytes cultured in palmitate (PA) and high D-glucose (HG) medium, mimicking that of a diabetic heart, exhibit suppressed autophagy, made evident by the decreased lipidated protein microtubule-associated protein 1A/1B-light chain 3 2 (LC3-II) and the elevated p62 levels, which are believed to be involved in autophagosome membrane expansion and autophagic flux, respectively [82]. Treatment of cardiomyocytes exposed to HG/PA with resveratrol results in p62 downregulation and LC3-II upregulation, indicating increased autophagic flux and enhanced autophagy [82]. Resveratrol treatment also decreases the amount of apoptosis induced by the HG/PA conditions. These two actions of resveratrol on the cells have been shown to be linked, as the autophagy inhibitor 3-methylademine (3-MA) diminishes RES’s effect on both processes [82]. Moreover, it has been shown that resveratrol exerts its effect on these processes via increasing phosphorylation of AMPK, thereby inhibiting the mTOR pathway and consequently increasing levels of LC3-II (). In addition, resveratrol actions seem to mediated by an increased phosphorylation of c-JunN-terminal kinase 1 (JNK1), which normally regulates the interaction between Beclin 1 and Bcl-2 [45]. Beclin 1 is a modulator of autophagosome formation [46], and Bcl-2 is a key anti-apoptotic factor. When Bcl-2 binds to Beclin 1, they inhibit each other’s functions and prevent autophagosome formation and apoptosis inhibition. JNK1 phosphorylates Bcl-2 [47], thereby interrupting the interaction between the two proteins and preserving their respective activities [48] (
-terminal kinase 1 (JNK1), which normally regulates the interaction between Beclin 1 and Bcl-2 [82]. Beclin 1 is a modulator of autophagosome formation [83], and Bcl-2 is a key anti-apoptotic factor [43]. When Bcl-2 binds to Beclin 1, they inhibit each other’s functions and prevent autophagosome formation and apoptosis inhibition. JNK1 phosphorylates Bcl-2 [84], thereby interrupting the interaction between the two proteins and preserving their respective activities [85] (Figure 2). Given that resveratrol increases JNK1 phosphorylation and thus Bcl-2 phosphorylation, its net effect is to increase autophagy and decrease apoptosis in HG/PA-treated cells [45]. Taken together, these results indicate a potential role for polyphenol use in vivo to reduce the deleterious effects of autophagy and apoptosis dysregulation on DCM.
). Given that resveratrol increases JNK1 phosphorylation and thus Bcl-2 phosphorylation, its net effect is to increase autophagy and decrease apoptosis in HG/PA-treated cells [82]. Taken together, these results indicate a potential role for polyphenol use in vivo to reduce the deleterious effects of autophagy and apoptosis dysregulation on DCM.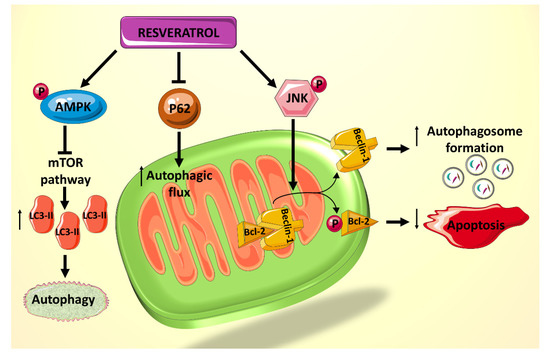
Figure 2.
6. Conclusions
There is undeniable evidence that polyphenols, particularly RES, play a significant role in hampering the progression and prognosis of DCM in animal models. They target multiple pathways that are central to the mitochondrial dysfunction associated with DCM and ameliorate the effects of a HG and lipid environment. These findings lay a strong foundation for future clinical investigations, and it would also be interesting to explore the effects of targeted resveratrol treatment on endoplasmic reticulum stress and its interaction with autophagy.
