SINS is a newly identified, distinct endogenous syndrome resulting from the combined presence and signs of inflammation and dead tissue in the acral areas. It particularly affects the tail base, tail tip, ears, coronary bands, heels, soles, claw walls, teats, navel, and face and can be observed in suckling piglets, weaners, and finishing pigs.
- tail biting
- inflammation and necrosis
- swine
- animal welfare
1. Introduction
Inflammation and loss of tail integrity can seriously impair animal welfare in pigs (European Food Safety Authority (EFSA) [1][2]. One of the most studied causes is tail biting, a very prevalent undesirable behaviour that has particularly been identified in growing pigs [3][4][5][6][7].
A number of external factors are well-accepted triggers for the problem, including: insufficient activity and boredom due to poor environmental enrichment and comfort; failure to satisfy natural behaviours; stress of any kind; low-quality air, food, and water; too much sunlight; excessive temperature; regrouping; excessive housing density; inappropriate pen structure, leading to confrontation between animals or the inability of all animals to feed simultaneously; disease; malnutrition; stimuli such as blood; and many more [7][8][9][10]. Internal factors such as aggressiveness, frustration, and genetic causes have also been identified as triggers for tail biting, and the multifactorial nature of the problem cannot be overcome by focusing on individual components [7][9]. Even with intensive use of the available measures, 25–70% of animals may have damaged tails (e.g., [11][12][13]). While earlier studies focused on tail-biting as a behavioural disorder attributable to the barren environment and housing conditions, a prevalence of tail biting between 14% and 20% was recorded in 2004 under extensive outdoor conditions, as is common practice in Switzerland [14].
Tail lesions are of special interest, not only due to their severe direct impact on animal welfare but, also, because tail docking is still used as the major preventive measure in most countries [15]. Tail docking, however, further increases damage, pain, and animal welfare concerns, without completely eliminating the problem and leaving the underlying causes unresolved [16]. Despite rising demands to ban tail docking in the EU (EU directive 2008/120IEC), field observations and scientific studies have shown that discontinuing tail docking under current practical conditions can seriously increase the prevalence of tail lesions [12][17].
Evidence from research and practice suggests that tail lesions might be caused not only by tail biting but, also, by inflammation and necrosis, which can occur without any action from other pigs [18][19][20][21][22][23][24][25][26]. In 50% of litters, up to 75% of piglets [19] can be affected. These lesions are also not limited to the tail but can be observed in the ears, heels and soles, claw coronary bands, teats, navel, vulva, and face [21][22][23][24][25][26]. The syndrome-like combination of different body parts and the clinical domination of inflammation and necrosis in these areas led to the term swine inflammation and necrosis syndrome (SINS; [22]).
The simultaneous occurrence in such disparate body regions as tail, teats, claws and others [22][24]; the evidence that SINS can be triggered before birth, when biting and mechanical irritation (e.g., from the floor) are excluded [25]; and the histopathological evidence for vascular-associated inflammation in neonatal piglets with (still) intact epidermis [23][24] all argue for a primary endogenous cause of the syndrome.
There is much to suggest that inflammation and lesions strongly affect animal welfare in swine, that they affect much more than just the tail of the animals and that tail biting and mechanical irritation due to technopathies alone are not sufficient to comprehensively address and combat the issue. The aim of this review is to summarize the current knowledge on SINS, to offer a hypothesis and mechanisms on the development of the syndrome and, derived from these, starting points to overcome the disease.
2. SINS Diagnostics and Differential Diagnostics
SINS signs are relatively easy to clinically diagnose. It is important to be aware of the meaning of the clinical signs and to clean the animals, e.g., in the area of the claws, in such a way that alterations can be detected. It is also important for scoring to be conducted by an experienced person and to expect variations between the results of different observers. However, no statements are available yet on the magnitude of the deviations that should be expected.
In SINS scoring, the base of the tail, the rest of the tail, including the tail tip, the ears, the teats, the navel, the coronary bands, the claw wall, the soles, and heels are considered [21][22][23]. The face and vulva can also be recorded. To facilitate scoring with a high repeatability of results and low stress to the piglets, the body parts to be assessed should be photographed. The actual scoring can then be done based on the photos [24][25].
Although it would be possible to score the individual body parts semi-quantitatively, binary scoring has proven to be effective so far. Here, an unaffected physiological state was scored with 0 and deviation with 1. This is simpler than semi-quantitative scoring and allows for a more comprehensible evaluation. Nevertheless, a good differentiation can be achieved by collecting the binary scores for all possible findings of the respective body parts (e.g., loss of bristles (0/1), swelling (0/1), reddening (0/1), rhagades (0/1), exudation (0/1), bleeding (0/1), necrosis (0/1), and ring-shaped constrictions (0/1)) for the tail (see below).
The tail and tail base are usually scored separately, considering the loss of bristles, swelling, reddening, rhagades, exudation, bleeding, clinical signs of necrosis, and the occurrence of ring-shaped constrictions at the tail (Figure 1A–I (top)). Ear scoring is focused on the loss of bristles, congestion of ear veins, and clinical necrosis. Each claw is individually scored for wall bulging, wall bleeding, sole reddening, detachment of sole from heel, reddening of heel, heel cracks, heel bleeding, detachment of heel, redness of coronary band, exudation from the coronary band, and clinical signs of necrosis in the coronary band (Figure 1A–D (middle bottom and bottom)). Field experience shows relatively good agreement between the findings of different claws.
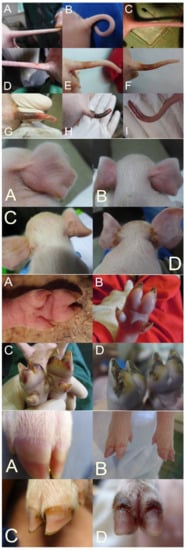
The different studies show some variation in the consideration of individual body parts and clinical variations. A precisely standardized procedure is not yet available. More studies are needed to define an optimal standard.
The resulting binary scores can be presented by the organ system as a percentage of the affected piglets. To ensure equal weighting of the organ scores, they can first be z-transformed and then added together. Again, exact standardization has not yet been performed, because possible weighting functions for the individual organ scores cannot yet be reasonably estimated. In addition, it is likely that optimal weighting functions will differ in different herds.
This can be difficult under practical conditions, especially in older piglets and fatteners, where mixed forms of bites, mechanical irritation due to technopathies, infection, and SINS might occur [23]. Tail biting has particularly been identified in growing pigs [3][4][5][6][7]. Tail biting cannot be responsible for tail lesions in piglets at birth, and suckling piglets have never been reported to bite their tails and, especially, not the tail base, where lesions are common in SINS [21][22][23][24][25]. The literature does not describe how annular tail lesions could result from bites or technopathies, nor how technopathies could lead to inflammation and lesions in the tail base area.
[27] associated ear necrosis with environmental factors and infections with Staphylococcus aureus and Staphylococcus hyicus. Papatsiros [28] [29] associated necrotic ear lesions with Treponema socranskii. [30] described a comparable syndrome, denoted PENS (porcine ear necrosis syndrome), in 5 to 10-week-old pigs.
Alopecia, swelling, coronary band injuries, and swelling and haemorrhaging into the claw corium were described in suckling piglets by Mouttotou et al. Recently, however, clear evidence for an additional internal component in such lesions was provided by histopathological findings in piglets [23]. Even in newborn piglets [24], 20.5%, 65.1%, 76%, and 82.9% of the individuals were found with the swelling of heels, inflammation of coronary bands, redness of heels, and wall bleeding, respectively, directly at birth. An ongoing study in these piglets is disclosing the histopathological findings in the claws and ears (Wenisch, pers.comm.).
Several results provide evidence that the expression of individual clinical signs of SINS is clearly modified by environmental effects. Pigs with SINS react more sensitively to unfavourable barn floor conditions than those with healthy claws due to the primary load in the area of the heels, soles, and claws. This explains why the signs of SINS can vary significantly depending on the existing environmental factors in different herds and why the correlations between organ scores can be relatively low [26]. However, in a cohort of 646 piglets, none was completely free from signs of SINS, and of the seven body parts examined, including the tail base, tail tip, face, ears, teats, navel, and claws, 3.8 ± 1.07 of body parts (mean ± SD) were affected simultaneously in an individual [25].
3. SINS as an Endogenous Disease
Three main observations support the assumption that SINS is primarily an endogenous disease, even though it may be modified by technopathies and other mechanical stressors: (1) The simultaneous occurrence in such disparate body parts as the tail, teats, claws [22][24][25]; (2) evidence that SINS can be expressed before birth [24]; (3) evidence that inflammation originating from blood vessels can be present before birth when biting and mechanical irritation (e.g., from soil) are excluded and in piglets with (still) intact epidermis [23][24].
Vasculitis, thrombosis, intimal proliferation, oedema, and hyperaemia were detected together with intact epidermis [23]. Bristle loss is associated with inflammatory processes in the deeper parts of hair follicles [23][24]. [24] on a conventional farm, 40–80% of neonatal piglets were affected by haemorrhages of the claw wall, coronal inflammation, redness of heels, bristle loss, and redness of the tail and ears. The inflammation could be characterized by granulocytes in considerable numbers, macrophages, and lymphocytes, indicating an onset of inflammation at least 4 days before birth [31], while the piglets were not older than 2 h.
Inflammation and necrosis have often been considered to result exclusively from biting and mechanical irritation. The authors suspected that inflammations and necroses were the result of circulatory disturbances, which were triggered by vasoconstriction and aggravated by further circumstances. This hypothesis was confirmed by the above-mentioned findings of vasculitis, intimal proliferation, and thrombus formation at the base of the tail, directly cranial to the clinical lesions with inflammation and necrosis [24]. In addition, several studies showed that inflammation of the tail tip (outside of biting events) is always associated with alterations at the tail base, while piglets with intact tail bases never show signs at the tail tip [23][24].
4. Hypothesis and Background of the Pathogenesis of SINS
[32], necrotic tail lesions in newborn piglets can be associated to the exposure of the sow to deoxynivalenol (DON). Additionally, the necrosis of tails, ears, and coronary bands in suckling piglets might be directly associated with mycotoxins and LPS (lipopolysaccharides) from sows’ milk [30][32][33][34][35][36]. To date, no accurate dose-response studies are available that allow precise quantification of the relationship between ingested mycotoxin and LPS levels and clinical consequences in piglets and pigs of different ages and against a background of different mixing ratios and cofactors. Nevertheless, among others, mycotoxins and LPS could be directly or indirectly implicated in causing the inflammation of blood vessels in affected body regions.
One of the most conclusive papers in this regard is that of Nordgreen et al. [37]. The authors suggested that problems in the microbiota, gut barrier, housing environment and hygiene, immune activation, mycotoxins, psychological stress, nutritional status, and feed composition can synergistically lead to inflammation directly or indirectly in association with LPS.
Abnormally high levels of degradation products from the gut are observed in the presence of increased microbial proliferation, intestinal disease, a high protein-to-crude fibre ratio, and disruption of the blood–intestinal barrier [38]. In pigs, damage to the blood–intestinal barrier directly results in increased LPS influx [39][40]. The blood–intestinal barrier in pigs is particularly susceptible to heat stress [41][42][43] and reduced gut perfusion with relative water deficiency and increased water requirements for thermoregulation [42][43] when contact cooling fails on dry concrete or plastic floors [44]. Mycotoxins (DON and similar substances) disintegrate the tight junctions of the blood–intestinal barrier and increase the LPS uptake in pigs [45][46].
Microbiota dysbiosis and intestinal barrier impairment are also associated with a number of chronic inflammatory disorders and systemic diseases in humans (for review, see references [47][48], and the pathogenic involvement of endotoxins is well-described [49][50][51].
However, in increased intestinal microbial proliferation, or if the intestinal barrier is disrupted (“leaky”), the liver is confronted with high concentrations of MAMPs. In this case, MAMPs are recognized not only by hepatic immune cells This results in liver inflammation and impaired organ functionality [52]. This could be explained by the crosstalk between inflammatory signalling (NF-κB, UPR) and hepatic lipid metabolic pathways in inflammatory liver diseases [53][54][55][56].
Pathogens, particularly of the digestive tract and respiratory tract, are common sources of MAMPs and frequent causes of elevated cytokine levels in pigs (reviewed in [37]). Poor hygiene, dust, LPS, and high levels of ammonia in the air are regularly present in pig houses and lead to activation of the inflammatory cascade via the respiratory tract [57][58][59][60]. Pigs are exposed to massive stressors, including high housing density, lack of pen structure, regrouping, lack of opportunities to eat at the same time, disease, poor housing air, etc. A number of studies demonstrate that cytokine activation comparable to that elicited by MAMPs is induced in humans [61], rodents [62], and, also, in pigs [63][64][65][66].
The pathogenicity of endotoxins in animals arises primarily from the release of endogenous mediators and is, thus, secondary [67]. However, the excessive or systemic activation of mediators by endotoxins can result in severe disease and possibly even death as a consequence of the mediator effect. Target cells for endotoxins are neutrophils, macrophages, and platelets. This mode of activation was also confirmed in pigs as the trigger for post-partum dysgalactia syndrome (PPDS) [68][69].
This leads to improved access for effector cells but, also, to tissue destruction. Thus, there is an overall increase in the influx of immunoglobulins and complement proteins, as well as immune cells, and lymphatic drainage is promoted. These changes also result in increased blood clotting; small vessels are relocated, and pathogens are prevented from spreading further in the organism. In addition to the cytokines already mentioned, biogenic amines (e.g., histamine and serotonin), oxygen radicals (NO and H2O2), and, mediated by cyclooxygenase-2 (COX-2) activity, various arachidonic acid derivatives, especially PGE2
The release of mediators occurs through activated defence cells (macrophages, Kupffer cells, etc.). Pathogens or their components invading via the intestine, urogenital tract, or fissures or wounds of the claws or skin are recognized by their MAMPs via specific receptors called Toll-like receptors (TLRs) in reference to the corresponding Toll receptors in Drosophila. The formation of this receptor complex activates a series of factors that introduce the signal that has arrived at the cell membrane into the cell and transfers it to the nucleus (overview in reference [70]). The final step is the formation of transcription factors—in particular, NF-κB, which starts their expression by binding in the promoter region of specific genes involved in inflammation, immune communication, and immunomodulation [38][71].
This leads to a reduction in tone and slackening of smooth muscle of blood vessels and the intestine. Prostaglandins have an activating and potentiating effect on macrophages and the immune system. Increased pain sensitivity develops at the nerve endings of pain fibres, and afferent fibres of the vagus nerve are irritated. Excessive production of the mediators results in the following symptoms: general depression, fever, impaired motility of the smooth muscles of the intestine (up to stasis) and the urogenital tract, and leukopenia, followed by leucocytosis, metabolic disturbances, and disturbances of the circulatory system up to shock.
Through prostaglandins (PG) and cytokines via the vagus nerve [72], there is a reporting of inflammatory processes to the central nervous system [73][74]. This can lead to systemic reactions such as depression, anorexia, and fever. The flooding of LPS from the intestinal tract leads to activation of the complement system. PGE2 travels via the blood pathway to the preoptic anterior hypothalamus (POA), where it triggers a fever via prostaglandin receptors.
Activation of corresponding centres of the area praeoptica by noradrenaline causes an induction of COX-2 there and, thus, also PGE2-mediated initiation of a fever. Within minutes after the recognition of bacterial components in the organism by macrophages, fever is formed [75][76]. The latter is realized both directly by the high temperature and by indirect effects, such as the lowering of various metal plasma levels required by bacteria at high temperatures. However, with increasing temperature, there are also more and more disruptions of important endogenous functions.
Disease sensations and disease-specific behaviour (sickness behaviour) eventually develop through the activation of PG receptors in the limbic system, with specific actions on ion channels [77]. The disease-specific sensation and behaviour that develop are not directly due to the fever but arise in parallel. These are highly organized behavioural changes after defence/immune stimulation, which are essential for dealing with pathogens, for example, in the sense of reserve conservation. In this way, inflammatory processes are associated with mental illness in humans and rodents (reviewed in reference [37]).
5. Genetic effects
Practical experience from pig farms with uniform sow base regularly shows evidence of boar or boar line effects on progeny SINS scores. A genetic basis of SINS has indeed been demonstrated [25]. Offspring from Duroc boars had significantly lower SINS scores than offspring from Pietrain boars, and within the Pietrain breed, SINS scores of offspring were significantly affected by the boar. Replacing the Pietrain boars by Duroc boars resulted in a 59% reduction in the SINS scores of their offspring under the given husbandry conditions. Total SINS scores in the offspring of the best Pietrain boars were almost 40% lower than that of offspring in the poorest Pietrain boars. These findings confirm considerable genetic effects on the outcome of SINS under a given husbandry. The results clearly show that individual breeding companies had boars with both favourable and unfavourable distribution of SINS in their offspring. It has to be assumed that the expression of the SINS signs is significantly influenced by husbandry and feeding. It can be suspected that the absolute differences between boars might be weaker or stronger under more favourable or less favourable conditions.
The genetics of the sow can also have significant effects on the SINS scores of the offspring [22]. Based on 20,000 pigs on 19 farms, it was found that more than 60% of fatteners from one of four sow lines (sows from four different relevant commercial breeding lines) had inflamed tails, while only 20-30% of fatteners from the other lines were affected. The same effect was observed for inflammation of the ears (40% vs. 0-13%). The fact that the sow line whose progeny showed high prevalence of tail and ear inflammation at the same time showed the least signs of biting, underlines the syndrome character of SINS on the one hand and proves on the other hand that tail lesions due to SINS or due to biting have to be strictly distinguished from each other. Of course, genetic differences a priori cannot be expected between all lines and populations.
6. Opportunities to improve SINS by environmental options
Improving water and raw fibre supplies is generally accepted to have a positive impact on intestinal health and the improvement of animal welfare in swine. Insufficient water uptake is a major risk factor for the development of postpartum dysgalactia syndrome (PPDS), a widespread disease in postpartal sows that is also related with inflammation triggered by MAMPs [78]. The resulting constipation is accompanied by exuberant bacterial growth in the intestine and flooding of endotoxins (MAMPs) [79][80]. This can be worsened by a lack of fibre [81][82], with the addition of fibre being “probably the most-cited factor to reduce PPDS” [83]. Constipation (coprostasis) is a leading sign and cause of PPDS]. Bacterial colonization of the endometrium, the bladder and the mamma were identified as further sources for MAMPs in PPDS [84]. Further resources for MAMPs were detected in injuries and fissures in teats and claws as well as laminitis in the sows [[80]]. Thus, sows with intact teats, claws, and skin, and which are free from coprostasis should have a lower burden on their piglets to develop SINS.
SINS scores in suckling piglets, weaners, and finishers of low-quality sows under standard housing conditions were highest but decreased significantly when housing conditions were improved. Sow quality had direct significant effects on inflammation and necrosis of suckling piglets and weaners under standard housing conditions. Offspring from sows with coprostasis had significantly higher SINS scores at every age. Improved housing resulted in a 39, 56, and 81% decrease in SINS symptomatology in suckling piglets, weaners, and finishers, respectively. The strongest effect of housing was the reduced incidence of coprostasis in sows. Thus, the husbandry effect could be split into a direct effect on the sows' offspring and an indirect effect by provoking coprostasis in the sow. The use of fibre, sanitized water from open drinkers, and sows with healthy claws, healthy teats, and intact skin can have positive effects on the prevalence of SINS in suckling piglets, weaners, and fatteners. Avoiding coprostasis in the sow seems to play a particularly important role [23].
7. Conclusions
Focusing on biting is only one part of the solution to control tail lesions in swine and includes three major concerns: 1) Tail damage can be found without any action by other pigs and up to 75% of piglets can be affected. 2) The lesions are not limited to the tail. They can also be found in ears, heels, soles, claws, coronary bands, teats, navel, vulva, and face. 3) Environmental improvement alone often fails to overcome the problem.
Recognizing a primary endogenous syndrome as the cause of clinically detectable in-flammation and necrosis at the aforementioned body parts leads to the second part of the solution: SINS. The syndrome can be triggered before birth and can be detected with con-siderable prevalence in neonates, suckling piglets, weaners, and fattening pigs.
The assumption that SINS is primarily an endogenous disease, even though it is modified by technopathies and other mechanical stressors, is supported by three findings: 1) The simultaneous occurrence of signs in disparate body parts such as the tail, teats, claws, and others; 2) the clinical expression of the syndrome before birth; 3) pathohisto-logical signs of vascular-associated inflammation with vasculitis and thrombosis, togeth-er with intact epidermis in newborn piglets, where biting and mechanical irritation (e.g., from the floor) can be excluded.
The idea of underlying circulatory disorders is supported by clinical and patho-histological results. A huge number of published findings support the hypothesis that these disorders might be due to microbe-associated molecular patterns (MAMPs), particularly from the intestine, that activate the defence cascade. The role of feed composi-tion, nutrition, mycotoxins, gut microbiota, gut barrier, housing environment, hygiene, immune activation, and even psychological stress has recently been elaborated in detail. The resulting expectation of liver inflammation with massive switch of metabolism from anabolism to acute phase and inflammation was demonstrated.
Using sows with intact claws, mammaries, and skin and which are free from consti-pation can favourably and sustainably influence inflammation and necrosis in their off-spring, from suckling piglets to finishing pigs. The use of crude fibre and sanitized water from open drinkers across all ages resulted in a further massive decrease in SINS signs at every age. Significant differences between offspring of Duroc and Pietrain boars and off-spring of different Pietrain boars suggest the possibility of a sustainable improvement of SINS by breeding.
References
- EFSA Panel on Animal Health and Welfare (AHAW). Statement on the use of animal-based measures to assess the welfare of animals. EFSA J. 2012, 10, 2767.
- EFSA Panel on Animal Health and Welfare (AHAW). Scientific Opinion concerning a multifactorial approach on the use of animal and non-animal-based measures to assess the welfare of pigs. EFSA J. 2014, 12, 3702.
- Heinonen, M.; Orro, T.; Kokkonen, T.; Munsterhjelm, C.; Peltoniemi, O.; Valros, A. Tail biting induces a strong acute phase response and tail-end inflammation in finishing pigs. Vet. J. 2010, 184, 303–307.
- Taylor, N.R.; Main, D.C.J.; Mendl, M.; Edwards, S.A. Tail-biting: A new perspective. Vet. J. 2010, 186, 137–147.
- Thodberg, K.; Herskin, M.S.; Jensen, T.; Jensen, K.H. The effect of docking length on the risk of tail biting, tail-directed behaviour, aggression and activity level of growing pigs kept under commercial conditions. Animal 2018, 12, 2609–2618.
- Ursinus, W.W.; Wijnen, H.J.; Bartels, A.C.; Dijvesteijn, N.; van Reenen, C.G.; Bolhuis, J.E. Damaging biting behaviors in intensively kept rearing gilts: The effect of jute sacks and relations with production characteristics. J. Anim. Sci. 2014, 92, 5193–5202.
- Nannoni, E.; Sardi, L.; Vitali, M.; Trevisi, E.; Ferrari, A.; Barone, F.; Bacci, M.L.; Barbieri, S.; Martelli, G. Effects of different enrichment devices on some welfare indicators of post-weaned undocked piglets. Appl. Anim. Behav. Sci. 2016, 184, 25–34.
- Breuer, K.; Sutcliffe, M.E.M.; Mercer, J.T.; Rance, K.A.; Beattie, V.E.; Sneddon, I.A.; Edwards, S.A. The effect of breed on the development of adverse social behaviours in pigs. Appl. Anim. Behav. Sci. 2003, 84, 59–74.
- Kallio, P.A.; Janczak, A.M.; Valros, A.E.; Edwards, S.A.; Heinonen, M. Case control study on environmental, nutritional and management-based risk factors for tail-biting in long-tailed pigs. Anim. Welf. 2018, 27, 21–34.
- D’Eath, R.B.; Arnott, G.; Turner, S.P.; Jensen, T.; Lahrmann, H.P.; Busch, M.E.; Niemi, J.K.; Lawrence, A.B.; Sandøe, P. Injurious tail biting in pigs: How can it be controlled in existing systems without tail docking? Animal 2014, 8, 1479–1497.
- Harley, S.; More, S.J.; O’Connell, N.E.; Hanlon, A.; Teixeira, D.L.; Boyle, L.A. Evaluating the prevalence of tail biting and carcase condemnations in slaughter pigs in the republic and Northern Ireland, and the potential of abattoir meat inspection as a welfare surveillance tool. Vet. Rec. 2012, 171, 621.
- Lahrmann, H.P.; Busch, M.E.; D’Eath, R.B.; Forkman, B.; Hansen, C.F. More tail lesions among undocked than tail docked pigs in a conventional herd. Animal 2017, 10, 1825–1831.
- Valros, A.; Välimäki, E.; Nordgren, H.; Vugts, J.; Fàbrega, E.; Heinonen, M. Intact tails as a welfare indicator in finishing pigs? Scoring of tail lesions and defining intact tails in undocked pigs at the abattoir. Front. Vet. Sci. 2020, 7, 405.
- Walker, P.; Bilkei, G. Tail-biting in outdoor pig production. Vet. J. 2006, 171, 367–369.
- Scollo, A.; Contiero, B.; Gottardo, E. Frequency of tail lesions and risk factors for tail biting in heavy pig production from weaning to 170 kg live weight. Vet. J. 2016, 207, 92–98.
- EFSA. Scientific opinion of the Panel on Animal Health and Welfare on a request from Commission on the risks associated with tail biting in pigs and possible means to reduce the need for tail docking considering the different housing and husbandry systems. EFSA J. 2007, 161, 1–13.
- Valros, A.; Ahlstrom, S.; Rintala, H.; Hakkinen, T.; Saloniemi, H. The prevalence of tail damage in slaughter pigs in Finland and associations to carcass condemnations. Acta Agric. Scand. Sect. A Anim. Sci. 2004, 54, 213–219.
- Penny, R.H.C.; Edwards, M.J.; Mulley, R. Clinical observations of necrosis of skin of suckling piglets. Aust. Vet. J. 1971, 47, 529–537.
- Blowey, R.; Done, S.H. Tail necrosis in pigs. Pig J. 2003, 51, 155–163.
- Santi, M.; Gheller, N.B.; Mores, T.J.; Marques, B.M.; Gonçalves, M.A.; Gava, D.; Zlotowski, P.; Driemeier, D.; Barcellos, D.E. Tail Necrosis in Piglets—Case Report. Available online: (accessed on 15 November 2018).
- Reiner, G.; Lechner, M. Inflammation and necrosis syndrome (SINS) in swine. CAB Rev. 2019, 14, 1–8.
- Reiner, G.; Lechner, M.; Eisenack, A.; Kallenbach, K.; Rau, K.; Müller, S.; Fink-Gremmels, J. Prevalence of an inflammation and necrosis syndrome in suckling piglets. Animal 2019, 13, 2007–2017.
- Reiner, G.; Kuehling, J.; Lechner, M.; Schrade, H.J.; Saltzmann, J.; Muelling, C.; Daenicke, S.; Loewenstein, F. Inflammation and Necrosis Syndrome is influenced by husbandry and quality of sow in suckling piglets, weaners and fattening pigs. Porc. Health Manag. 2020, 6, 32.
- Kuehling, J.; Loewenstein, F.; Wenisch, S.; Kressin, M.; Herden, C.; Lechner, M.; Reiner, G. An in-depth diagnostic exploration of an inflammation and necrosis syndrome in a population of newborn piglets. Animal 2021, 15, 100078.
- Kuehling, J.; Eisenhofer, K.; Lechner, M.; Becker, S.; Willems, H.; Reiner, G. The effects of boar on susceptibility to swine inflammation and necrosis syndrome in piglets. Porc. Health Manag. 2021, 7, 15.
- Ringseis, R.; Gessner, D.; Löwenstein, F.; Kuehling, J.; Becker, S.; Willems, H.; Lechner, M.; Eder, K.; Reiner, G. Swine inflammation and necrosis syndrome is associated with plasma metabolites and liver transcriptome in affected piglets. Animals 2021, 11, 772.
- Park, J.; Friendschip, R.M.; Poliak, Z.; DeKay, D.; Slavic, D. An investigation of ear necrosis in pigs. Can. Vet. J. 2013, 54, 491–495.
- Papatsiros, V. Ear necrosis syndrome in weaning pigs associated with PCV2 infection: A case report. Vet. Res. 2012, 3, 217–220.
- Pringle, M.; Backhans, A.; Otman, F.; Sjölund, M.; Fellström, C. Isolation of spirochetes of genus Treponema from pigs with ear necrosis. Vet. Microbiol. 2009, 18, 279–283.
- Weissenbacher-Lang, C.; Voglmayr, T.; Waxenecker, F.; Hofstetter, U.; Weissenböck, H.; Hoelzle, K.; Ritzmann, M. Porcine ear necrosis syndrome: A preliminary investigation of putative infectious agents in piglets and mycotoxins in feed. Vet. J. 2012, 194, 392–397.
- Betz, P. Histological and enzyme histochemical parameters for the age estimation of human skin wounds. Int. J. Leg. Med. 1994, 107, 60–68.
- Van Limbergen, T.; Devreese, M.; Croubels, S.; Broekaert, N.; Michiels, A.; DeSaeger, S.; Maes, D. Role of mycotoxins in herds with and without problems with tail necrosis in neonatal pigs. Vet. Rec. 2017, 181, 539.
- Schrauwen, E.; Thoonen, H.; Hoorens, J.; Houvenaghel, A. Pathophysiological effects of endotoxin infusion in young piglets. Br. Vet. J. 1986, 142, 364–370.
- Jadamus, A.; Schneider, D. Long-term effect of fusariotoxins on the reproduction performance of sows testing the effectiveness of detoxifying feed additives 700. Feed Mag. 2002, 10, 396–405.
- Busch, M.E.; Jensen, I.M.; Korsgaard, J. Development and consequences of ear necrosis in a weaner herd and two growing-finishing herds. In Proceedings of the 21st International Pig Veterinary Society Congress, Vancouver, BC, Canada, 18–21 July 2010; p. 45.
- Guillou, D.; Demey, V.; Chacheyras-Durand, F.; Le Treut, Y. Mise en evidence du transfer des endotoxines de la truie vers sa portée dans le context du syndrome de dysgalactie post-partum. J. Rech. Porc. 2013, 45, 269–270.
- Nordgreen, J.; Edwards, S.A.; Boyle, L.A.; Bolhuis, J.E.; Veit, C.; Sayyari, A.; Marin, D.E.; Dimitrov, I.; Janczak, A.M.; Valros, A. A proposed role for proinflammatory cytokines in damaging behavior in pigs. Front. Vet. Sci. 2020, 7, 646.
- Klein, K.; Fuchs, G.J.; Kulanpongs, P.; Mertz, G.; Suskind, R.M.; Olson, R.E. Endotoxemia in protein-energy malnutrition. J. Ped. Gastroenterol. Nutr. 1988, 7, 225–228.
- Hunt, K.M.; Brooker, S.L.; Sanz-Fernandez, M.V.; Gabler, N.K.; Baumgard, L.H.; McGuire, M.A. The effects of heat stress and Zn intake on the microbial communities in the stomach, ileum, colon and feces of pigs. FASEB J. 2013, 27, 356.7. Available online: (accessed on 3 June 2021).
- Sanz Fernandez, M.V.; Stoakes, S.K.; Abuajamieh, M.; Seibert, J.T.; Johnson, J.S.; Horst, E.A.; Rhoads, R.P.; Baumgard, L.H. Heat stress increases insulin sensitivity in pigs. Physiol. Reprod. 2015, 3, e1247.
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Baumgard, L.H.; Gabler, N.K. Heat stress reduces barrier function and alters intestinal metabolism in growing pigs. J. Anim. Sci. 2012, 90, 257–259.
- Pearce, C.S.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Gabler, N.K. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS ONE 2013, 8, e70215.
- Pearce, S.C.; Sanz-Fernandez, M.V.; Hollis, J.H.; Baumgard, L.H.; Gabler, N.K. Short-term exposure to heat stress attenuates appetite and intestinal integrity in growing pigs. J. Anim. Sci. 2014, 92, 5444–5454.
- Rudovsky, A. Anforderungen an die Stallfußböden für die Schweinehaltung. Bau. Landwirtsch. 2001, 3, 5–7.
- Alizadeh, A.; Braber, S.; Akbari, P.; Kraneveld, A.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol and its modified forms: Are there major differences? Toxins 2016, 8, 334.
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of two mycotoxins deoxynivalenol and fumonisin on pig intestinal health. Porc. Health Manag. 2016, 2, 21.
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189.
- Yu, L.C.H. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: Exploring a common ground hypothesis. J. Biomed. Sci. 2018, 25, 79.
- Brandl, K.; Schnabl, B. Is intestinal inflammation linking dysbiosis to gut barrier dysfunction during liver disease? Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1069–1076.
- Carotti, S.; Guarino, M.P.L.; Vespasiani-Gentilucci, U.; Morini, S. Starring role of toll-like receptor-4 activation in the gut-liver axis. World, J. Gastrointest. Pathophysiol. 2015, 6, 99–109.
- Bowser, S.M.; McMillan, R.P.; Boutagy, N.E.; Tarpey, M.D.; Smithson, A.T.; Osterberg, K.L.; Neilson, A.P.; Davy, B.M.; Davy, K.P.; Hulver, M.W. Serum endotoxin, gut permeability and skeletal muscle metabolic adaptations following a short term high fat diet in humans. Metab. Clin. Exp. 2020, 103, 154041.
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145.
- Puri, P.; Wiest, M.M.; Patnaik, M.; Cheung, O.; Sargeant, C.C.; Mirshahi, F.; Watkins, S.M.; Sanyal, A.Y. The plasma lipidomic signature of nonalcoholic steatohepatitis: Differential levels of n-3 and n-6 polyunsaturated fatty acids and their lipoxygenase products. Hepatology 2007, 46, 310A–311A.
- Allard, J.; Aghdassi, E.; Mohammed, S.; Raman, M.; Avand, G.; Arendt, B.M.; Jalali, P.; Kandasamy, T.; Prayitno, N.; Sherman, M.; et al. Nutritional assessment and hepatic fatty acid composition in non-alcoholic fatty liver disease (NAFLD): A cross-sectional study. J. Hepatol. 2008, 48, 300–307.
- Chiappini, F.; Coilly, A.; Kadar, H.; Gual, P.; Tran, A.; Desterke, C.; Samuel, D.; Duclos-Vallee, J.C.; Touboul, D.; Bertrand-Michel, J.; et al. Metabolism dysregulation induces a specific lipid signature of nonalcoholic steatohepatitis in patients. Sci. Rep. 2017, 7, 46658.
- Song, M.J.; Malhi, H. The unfolded protein response and hepatic lipid metabolism in non-alcoholic fatty liver disease. Pharmacol. Ther. 2019, 203, 107401.
- Murata, H.; Horino, R. Effects of in vitro atmospheric ammonia exposure on recovery rate and luminol-dependent chemiluminescence of bovine neutrophils and bronchoalveolar macrophages. J. Vet. Med. Sci. 1999, 61, 279–281.
- Van Gucht, S.; Van Reeth, K.; Pensaert, M. Interaction between porcine reproductive-respiratory syndrome virus and bacterial endotoxin in the lungs of pigs: Potentiation of cytokine production and respiratory disease. J. Clin. Microbiol. 2003, 41, 960–966.
- Von Borell, E.; Eslinger, K.M.; Schnitz, A.L.; Zhao, Y.; Mitloehner, F.M. Acute and Prolonged Effects of Ammonia on Hematological Variab Les, Stress Responses, Performance, and Behavior of Nursery Pigs. J. Swine Health Prod. 2007, 15, 137–145.
- Roque, K.; Shin, K.M.; Jo, J.H.; Lim, G.D.; Song, E.S.; Shin, S.J.; Heo, Y. Association between endotoxin levels in dust from indoor swine housing environments and the immune responses of pigs. J. Vet. Sci. 2018, 19, 331–338.
- Maes, M.; Song, C.; Lin, A.; De Jongh, R.; Van Gastel, A.; Kenis, G.; Smith, R.S. The effects os psychological stress on humans: Increased production of proinflammatory cytokines and a TH1-like response in stress-induced anxiety. Cytokine 1998, 10, 313–318.
- Zhao, X.N.; Cao, F.R.; Liu, Q.; Li, X.S.; Xu, G.Y.; Liu, G.; Ma, J. Behavioral, inflammatory and neurochemical disturbances in LPS and UCMS-induced mouse models of depression. Behav. Brain Res. 2019, 364, 494–502.
- Kantiz, E.; Tuchscherer, M.; Puppe, B.; Tuchscherer, A.; Stabenow, B. Consequences of repeated early isolation in domestic piglets (Sus scrofa) on their behavioral, neuroendocrine, and immunological responses. Brain Behav. Immun. 2004, 18, 35–45.
- Gimsa, U.; Tuchscherer, M.; Kanitz, E. Psychosocial stress and immunitywhat can we learn from pig studies? Front. Behav. Neurosci. 2018, 12, 64.
- Tuchscherer, M.; Kanitz, E.; Puppe, B.; Tuchscherer, A.; Stabenow, B. Effects of postnatal social isolation on hormonal and immune responses of pigs to an acute endotoxin challenge. Physiol. Behav. 2004, 82, 503–511.
- Tuchscherer, M.; Puppe, B.; Tuchscherer, A.; Kanitz, E. Psychosocial stress sensitizes neuroendocrine and inflammatory responses to Escherichia coli challenge in domestic piglets. Brain Behav. Immun. 2018, 68, 274–287.
- Smith, B.B. Understanding the role of endotoxins in gram negative septicaemia. Vet. Med. 1986, 17, 1148–1161.
- Nachreiner, R.F.; Ginther, O.J. Induction of agalactia by administration of endotoxin (Escherichia coli) in swine. Am. J. Vet. Res. 1974, 35, 619–622.
- Zhu, Y.; Fossum, C.; Berg, M.; Magnusson, U. Morphometric analysis of pro-inflammatory cytokines in mammary glands of sows suggest an association between clinical mastitis and local production of IL-1 beta, IL-6 and TNFalpha. Vet. Res. 2007, 38, 871–882.
- Heumann, D.; Roger, T. Initial responses to endotoxins and Gram-negative bacteria. Clin. Chim. Acta 2002, 323, 59–72.
- Papatsiros, V.G.; Alexopoulos, C.; Kyriakis, S.C. Latest information in relation to Postpartum Dysgalactia Syndrome of sows. J. Hell. Vet. Med. Soc. 2007, 58, 61–75.
- Bluthe, R.M.; Walter, V.; Parnet, P.; Laye, S.; Lestage, J.; Verrier, D.; Dantzer, R. Lipopolysaccharide induces sickness behavior in rats by a vagal mediated mechanism. Comptes Rendus Acad. Sci. III 1994, 317, 499–503.
- Quan, N.; Whiteside, M.; Herkenham, M. Time course and localization patterns of interleukin-1 beta messenger RNA expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience 1998, 83, 281–293.
- Vitkovic, L.; Konsman, J.P.; Bockaert, J.; Dantzer, R.; Homburger, V.; Jacque, C. Cytokine signals propagate through the brain. Mol. Psychiatry 2000, 5, 604–615.
- Boulant, J.A. Hypothalamic mechanisms in thermoregulation. Fed. Proc. 1981, 40, 2843–2850.
- Boulant, J.A. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin. Infect. Dis. 2000, 31 (Suppl. 5), 157–161.
- Haba, R.; Shintani, N.; Onaka, Y.; Kanoh, T.; Wang, H.; Takenaga, R.; Hayata, A.; Hirai, H.; Nagata, K.; Nakamura, M. Central CRTH2, a Second Prostaglandin D-2 Receptor, Mediates Emotional Impairment in the Lipopolysaccharide and Tumor-Induced Sickness Behavior Model. J. Neurosci. 2014, 34, 2514–2523.
- Reiner, G.; Hertrampf, B.; Richard, H.R. Postpartales Dysgalaktiesyndrom der Sau—Eine Übersicht mit besonderer Berücksichtigung der Pathogenese. Tierarztl. Prax. 2009, 5, 305–318.
- Hermansson, I.; Einarsson, S.; Larson, K.; Bäckström, L. On the agalactia postpartum in the sow: A clinical study. Nord. Vet. 1978, 30, 465–473.
- Hirsch, A.C.; Philipp, H.; Kleemann, R. Investigation on the efficacy of meloxicam in sows with mastitis‐metritis‐agalactia syndrome. J. Vet. Pharmocol. Ther. 2003, 26, 355–360.
- Smith, B.B.; Wagner, W.C. Effect of dopamine agonists or antagonists, TRH, stress and piglet removal on plasma prolactin concentrations in lactating gilts. Theriogenology 1985, 3, 283–296.
- Smith, B.B.; Wagner, W.C. Effect of Escherichia coli endotoxin and thyrotropin releasing hormone on prolactin in lactating sows. Am. J. Vet. Res. 1985, 46, 175–180.
- Martineau, G.; Farmer, C.; Peltoniemi, O. Mammary System. In Diseases of Swine, 4th ed.; Zimmermann, J., Karriker, L., Ramirez, A., Schwartz, K., Stevenson, G., Eds.; Wiley: West Sussex, UK, 2012; pp. 270–293.
- Holst, H.; Kindahl, H. Hematological and blood biochemical effects of fasting and subsequent oral administration of endotoxin in prepubertal gilts. Acta. Vet. Scand. 1995, 36, 499–508.
