Interstitial Lung Diseases (ILDs) represent a heterogeneous group of pathologies, which may be related to different causes. A low percentage of these lung diseases may be secondary to the administration of drugs or substances. Several different high resolution computed tomography (HRCT) patterns related to pulmonary drug toxicity have been reported in literature, and the most frequent ILDs patterns reported include Nonspecific Interstitial Pneumonia (NSIP), Usual Interstitial Pneumonia (UIP), Hypersensitivity Pneumonitis (HP), Organizing Pneumonia (OP), Acute Respiratory Distress Syndrome (ARDS), and Diffuse Alveolar Damage (DAD).
- lung diseases
- interstitial
- multidetector computed tomography
- idiopathic pulmonary fibrosis
- toxicity
- respiratory distress syndrome
- acute
1. Introduction
| Drugs | Estimated Incidences | References | |||
|---|---|---|---|---|---|
| References | |||||
| Nitrofurantoina | 1 on 5000 (acute toxicity) | [8] | |||
| OP | Amphotericin-B, Amiodarone, Bleomycin, Doxorubicin, Interferon, Metotrexatem, Mitomycin, Nitrofurantonina, Phenytoin, Ticlopidine, Tryptophan, Sulphalazine | [14] | |||
| Acetyl-salicylic acid | From 4% (general adult population) to 25% (asthmatic patients) | ||||
| HP | Ampicillin, Bupropion, Carbamazepine, Ciprofloxacin, Citarabine, Cephalosporins, interferon-alpha, sulfonamides, ticlopidine, trimethoprim-sulfamethoxazole, sirolimus | [9] | |||
| [ | 9 | ] | Amiodarone | 6% | [10] |
| Interstitial pneumonia | Adalimumab, Amphotericin B, Amiodarone, Azathioprine, Bleomycin, Busulfan, Chlorambucil, Cyclofosphamide, Etanercept, Flecainide, Interferon alfa, Interferon beta, Infliximab, Melphalan, Methadone, Metotrexate, Nitrofurantoin, Paclitaxel, Penicillamine, Rituximab, Sirolimus, Statine, Sulfasalazine | [14] | Methotrexate | 7% (chronic toxicity), very rare (acute toxicity) | |
| Loeffler syndorme | Amiodarone, ASA, Bleomycin, Carbamazepine, Captopril, Ibuprofen, Imipramine, Isoniazide, Metotrexate, GM-CSF, Naproxen, Gold salts, Sulfasalazine, Procarbazine, Penicillins, Tryptophans, Zafirleukast | [11] | |||
| [ | 11 | ] | Bleomycin | 10% | |
| Pulmonary edema | Amlodipine, ASA, Cyclosporine, Citarabine, Chlorothiazide, Clozapine, Heroin, Epinephrine, Gemcitabine, Ketoprofen, Interleukin, Methadone, Metotrexate, Mitomycin, Nitric Oxide, Propanolol, Verapamil | [12] | |||
| [ | 14 | ] | Busulfan | 4% | |
| ARDS | Amiodarone, Citarabine, Immunoglobulins, GM-CSF, Nitrofurantoin, Infliximab, Talc, Vinblastine, Vincristine | [9] | |||
| [ | 14 | ] | Mitomycin | 2–38% | [13] |
| Cyclofosphamide | 1% (when used as single agent) | [9] |
| Pattern | Associated Drugs |
|---|
2. Etiopathogenesis and Risk Factors
3. Antibiotics
4. Anti-Inflammatory and Immunomodulators Drugs
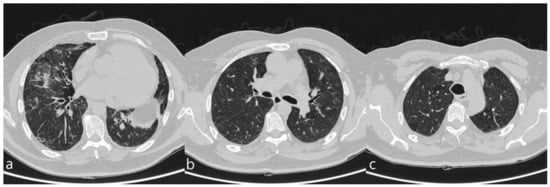
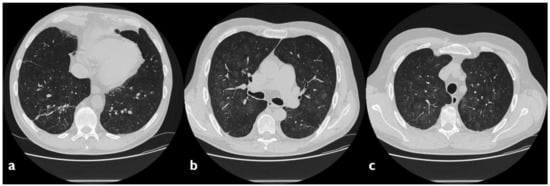
5. Anti-Neoplastic Drugs
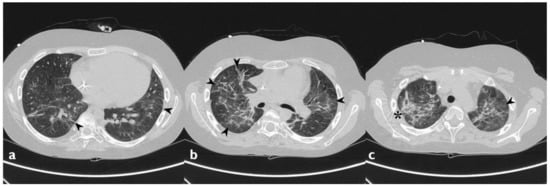
6. Diagnosis
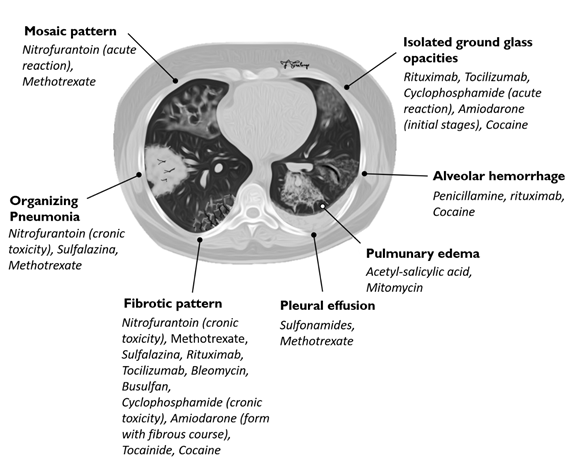
The diagnosis of DILDs is not simple and requires the integration of clinical, laboratory and imaging data. Histological examination demonstrates substantially identical characteristics between idiopathic and drug-related forms; physical, laboratory and radiological findings are not able, separately, to resolve the diagnostic questions. HRCT appears to be the most sensitive radiological examination to make a diagnosis of interstitial disease, but it should be performed and evaluated by radiologists with experience in pulmonary diseases and in a multidisciplinary approach (Table 3, Figure 4); however, the diagnosis of drug toxicity remains a diagnosis by exclusion, and other concomitant interstitial diseases or infectious diseases must be taken into consideration. In this rather complex and uncertain context, the resolution of diagnostic doubt is represented by the possible cause‐effect relationship between the onset of a pulmonary pathology and exposure to a drug; it is therefore useful to evaluate the recent pharmacological anamnesis and investigate any previous ones.
Table 3. Association between HRCT patterns and the drugs most frequently responsible for lung toxicity.
|
HRCT Pattern |
Associated drugs |
|
Fibrotic pattern |
Nitrofurantoin (chronic toxicity), methotrexate, sulfalazina, rituximab, tocilizumab, bleomycin, busulfan, cyclophosphamide (chronic toxicity), amiodarone (form with fibrous course), tocainide, cocaine |
|
Organizing pneumonia |
Nitrofurantoin (chronic toxicity), methotrexate |
|
Mosaic pattern |
Nitrofurantoin (acute toxicity), methotrexate, sulfalazina |
|
Isolated ground glass |
Rituximab, tocilizumab, cyclophosphamide (acute reaction), amiodarone (initial stage), cocaine |
|
Alveolar hemorrhage |
Penicillamine, rituximab, cocaine |
|
Pulmonary edema |
Acetyl-salicylic acid, mitomycin |
|
Pleural effusion |
Sulfonamides, methotrexate |
Figure 4. Association between HRCT patterns and the drugs most frequently responsible for lung toxicity.
7. Management
In acute form, the suspension of the injurious drug should be considered in favor of substitution with another drug. The suspect drug should be avoided in future treatments. In chronic forms, the fibrotic lesions are essentially irreversible, regardless of the suspension of the drug. In OP and HP, cortisone may play a role in the correction of symptoms and in accelerating the resolution of the clinical picture.
References
- Cleverley, J.R.; Screaton, N.J.; Hiorns, M.P.; Flint, J.D.; Müller, N.L. Drug-induced lung disease: High-resolution CT and histological findings. Clin. Radiol. 2002, 57, 292–299.
- Lateef, O.; Shakoor, N.; Balk, R.A. Methotrexate pulmonary toxicity. Expert Opin. Drug Saf. 2005, 4, 723–730.
- Edwards, I.R.; Aronson, J.K. Adverse drug reactions: Definitions, diagnosis, and management. Lancet 2000, 356, 1255–1259.
- Pirmohamed, M.; James, S.; Meakin, S.; Green, C.; Scott, A.K.; Walley, T.J.; Farrar, K.; Park, B.K.; Breckenridge, A.M. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18,820 patients. BMJ 2004, 329, 15–19.
- Farcas, A.; Sinpetrean, A.; Mogosan, C.; Palage, M.; Vostinaru, O.; Bolita, M.; Domitrascu, D. Adverse drug reactions detected by stimulated spontaneous reporting in an internal medicine department in Romania. Eur. J. Intern. Med. 2010, 21, 453–457.
- Giardina, C.; Cutroneo, P.M.; Mocciaro, E.; Russo, G.T.; Mandraffino, G.; Basile, G.; Rapisarda, F.; Ferrara, R.; Spina, E.; Arcoraci, V. Adverse Drug Reactions in Hospitalized Patients: Results of the FORWARD (Facilitation of Reporting in Hospital Ward) Study. Front. Pharmacol. 2018, 9, 350.
- Thomeer, M.J.; Costabe, U.; Rizzato, G.; Poletti, V.; Demedts, M. Comparison of registries of interstitial lung diseases in three European countries. Eur. Respir. J. Suppl. 2001, 18 (Suppl. 32), 114s–118s.
- Kabbara, W.K.; Kordahi, M.C. Nitrofurantoin-induced pulmonary toxicity: A case report and review of the literature. J. Infect Public Health 2015, 8, 309–313.
- Bennoun, L. Drug induced respiratory disorders. Drug Safety 2000, 23, 143–164.
- Dean, P.J.; Groshart, K.D.; Porterfield, J.G. Amiodarone-associated pulmonary toxicity: A clinical and pathologic study of eleven cases. Am. J. Clin. Pathol. 1987, 87, 7–13.
- Jackevicius, C.A.; Tom, A.; Essebag, V.; Eisenberg, M.J.; Rahme, E.; Tu, J.V.; Humphries, K.; Behlouli, H.; Pilote, L. Population-level incidence and risk factors for pulmonary toxicity associated with amiodarone. Am. J. Cardiol. 2011, 108, 705–710.
- Jules-Elysee, K.; White, D. Bleomycin induced pulmonary toxicity. Clin. Chest Med. 1990, 11, 1–20.
- Linette, D.C.; McGee, K.H.; McFarland, J.A. Mitomycin-induced pulmonary toxicity: Case report and review of the literature. Ann.Pharmacother. 1992, 26, 481–484.
- Dweik, R.A. Drug-induced pulmonary disease. In Textbook of Pulmonary Diseases, 6th ed.; Baum, G.L., Crapo, J.D., Celli, B.R., Karlinsky, J.B., Eds.; Lippincott Raven: Philadelphia, PA, USA, 1988; pp. 477–490.
- Schwaiblmair, M.; Behr, W.; Haeckel, T.; Markl, B.; Foerg, B.; Berghaus, T. Drug induced interstitial lung disease. Open Respir. Med. J. 2012, 6, 63–74.
- Simpson, A.B.; Paul, J.; Graham, J.; Kaye, S.B. Fatal bleomycin pulmonary toxicity inthe west of Scotland 1991–95: A review of patients with germ cell tumours. Br. J. Cancer 1998, 78, 1061–1066.
- Wijnen, P.A.; Drent, M.; Nelemans, P.J.; Kuijipers, P.M.; Koek, G.H.; Neef, C.; Haenen, G.R.; Bekers, O. Role of cytochrome P450 polymorphisms in the development of pulmonary drug toxicity: A case-control study in the Netherlands. Drug Saf. 2008, 31, 1125–1134.
- Park, B.L.; Kim, T.H.; Kim, J.H.; Bae, J.S.; Pasaje, C.F.; Cheong, H.S.; Kim, L.H.; Park, J.S.; Lee, H.S.; Kim, M.S.; et al. Genome-wide association study of aspirin-exacerbated respiratory disease in a Korean population. Hum Genet. 2013, 132, 313–321.
- Roden, A.C.; Camus, P. Iatrogenic pulmonary lesions. Semin. Diagn. Pathol. 2018, 35, 260–271.
- Giridhar, P.; Mallick, S.; Rath, G.K.; Julka, P.K. Radiation induced lung injury: Prediction, assessment and management. Asian Pac. J. Cancer Prev. 2015, 16, 2613–2617.
- Stemmer, S.M.; Cagnoni, P.J.; Shpall, E.J.; Bearman, S.I.; Matthes, S.; Dufton, C.; Day, T.; Taffs, S.; Hami, L.; Martinez, C.; et al. High-dose paclitaxel, cyclophosphamide, and cisplatin with autologous hematopoietic progenitor-cell support: A phase I trial. J. Clin. Oncol. 1996, 14, 1463–1472.
- Yamada, Y.; Shiga, T.; Matsuda, N.; Hagiwara, N.; Kasanuki, H. Incidence and predictors of pulmonary toxicity in Japanese patients receiving low-dose amiodarone. Circ. J. 2007, 71, 1610–1616.
- Kudoh, S.; Kato, H.; Nishiwaki, Y.; Fukuoka, M.; Nakata, K.; Ichinose, Y.; Tsuboi, M.; Yokota, S.; Nakagawa, K.; Suga, M.; et al. Interstitial lung disease in Japanese patients with lung cancer: A cohort and nested case-control study. Am. J. Respir. Crit. Care Med. 2008, 177, 1348–1357.
- Sathi, N.; Chikura, B.; Kaushik, V.V.; Kaushik, V.V.; Wiswell, R.; Dawson, J.K. How common is methotrexate pneumonitis? A large prospective study investigates. Clin. Rheumatol. 2012, 31, 79–83.
- Suissa, S.; Hudson, M.; Ernst, P. Leflunomide use and the risk of interstitial lung disease in rheumatoid arthritis. Arthritis Rheum. 2006, 54, 1435–1439.
- Skeoch, S.; Weatherley, N.; Swift, A.J.; Oldroyd, A.; Johns, C.; Hayton, C.; Giollo, A.; Wild, J.M.; Waterton, J.C.; Buch, M.; et al. Drug-Induced Interstitial Lung Disease: A Systematic Review. J. Clin. Med. 2018, 7, 356.
- Padley, S.P.G.; Adler, B.; Hansell, D.M.; Müller, N.L. High-resolution computed tomography of drug-induced lung disease. Clin. Radiol. 1992, 46, 232–236.
- Wang, K.K.; Bowyer, B.A.; Fleming, C.R.; Schroeder, K.W. Pulmonary infiltrates and eosinophilia associated with sulphasalazine. Mayo Clin. Proc 1984, 59, 343–346.
- Zitnik, R.J.; Cooper, J.A., Jr. Pulmonary disease due to antirheumatic agents. Clin. Chest Med. 1990, 11, 139–150.
- Ohbayashi, M.; Suzuki, M.; Yashiro, Y.; Fukuwaka, S.; Yasuda, M.; Kohyama, N.; Kobayashi, Y.; Yamamoto, T. Induction of pulmonary fibrosis by methotrexate treatment in mice lung in vivo and in vitro. J. Toxicol. Sci. 2010, 35, 653–661.
- Prasad, R.; Gupta, P.; Singh, A.; Goel, N. Drug induced pulmonary parenchymal disease. Drug Discov. Ther. 2014, 8, 232–237.
- Peerzada, M.M.; Spiro, T.P.; Daw, H.A. Pulmonary toxicities of biologics: A review. Anticancer Drugs. 2010, 21, 131–139.
- Hadjinicolaou, A.V.; Nisar, M.K.; Bhagat, S.; Parfey, H.; Chilvers, E.R.; Ostor, A.J. Non-infectious pulmonary complications of newer biological agents for rheumatic diseases—A systematic literature review. Rheumatology (Oxford) 2011, 50, 2297–2305.
- Zayen, A.; Rais, H.; Rifi, H.; Ouarda, M.; Afrit, M.; Cherif, A.; Mezline, A. Rituximab-induced interstitial lung disease: Case report and literature review. Pharmacology 2011, 87, 318–320.
- Sekimoto, Y.; Kato, M.; Shukuya, T.; Kouama, R.; Nagaoka, T.; Takahashi, K. Bevacizumab-induced chronic interstitial pneumonia during maintenance therapy in non-small cell lung cancer. Respirol. Case Rep. 2016, 4, e00151.
- Radzikowska, E.; Szczepulska, E.; Chabowski, M.; Bestry, I. Organising pneumonia caused by transtuzumab (Herceptin) therapy for breast cancer. Eur. Respir. J. 2003, 21, 552–555.
- Romond, E.H.; Perez, E.A.; Bryant, J.; Surnan, V.J.; Geyer, C.E.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Soonmyung, D.O.; Kaufman, P.A.; et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Eng. J. Med. 2005, 353, 1673–1684.
- Hadjinicolaou, A.V.; Nisar, M.K.; Parfrey, H.; Chilvers, E.R.; Ostör, A.J. Non-infectious pulmonary toxicity of rituximab: A systematic review. Rheumatology (Oxford) 2012, 51, 653–662.
- Segura, A.; Yuste, A.; Cercos, A.; López-Tendero, P.; Gironés, R.; Pérez-Fidalgo, J.A.; Herranz, C. Pulmonary fibrosis induced by cyclophosphamide. Ann. Pharmacother. 2001, 35, 894–897.
- Malik, S.W.; Myers, J.L.; DeRemee, R.A.; Specks, U. Lung toxicity associated with cyclophosphamide use. Two distinct patterns. Am. J. Respir. Crit. Care Med. 1996, 154, 1851–1856.
