cAMP was first discovered in 1958 and introduced the concept of a “second messenger” system. In fact, this molecule, together with cyclic guanosine monophosphate (cGMP), has been identified as an important intracellular translator of membrane signaling originating from hormones, growth factors, cytokines and other molecules. In the general transduction mechanism, the stimulated receptor activates the corresponding G-coupled protein, leading to increased adenylyl cyclase-mediated cAMP synthesis.
- cholangiocarcinoma
- cAMP
- cholangiocytes
- proliferation
- PKA
- secretin
1. Introduction
The biliary tree is lined by epithelial cells (i.e., cholangiocytes), which appear to originate from a common stem cell compartment, the hepatic progenitor cells, similar to hepatocytes [1]. These pluripotent cells are located at the interface between the hepatocyte canaliculi and bile ductules in the canals of Hering (Figure 1), the latter being the smaller branches of the biliary tree [2].
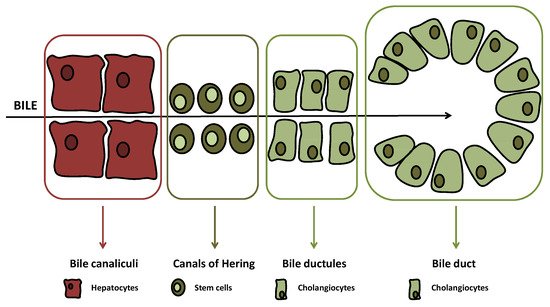
(≤15 μm in diameter) or large ducts (≥15 μm in diameter) While, at the beginning, just bile duct secretory activities were studied with regard to biliary tract diseases, lately also, biliary proliferative phenotypes have gained interest [3][4][5][10,11,12]. For instance, atypical cholangiocyte proliferation, characterized by truncated tortuous bile ducts, has been observed in adult cholestatic liver diseases such as primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC) [6][7][13,14]. Both diseases are defined as ductopenia, since changes and evolution of proliferation determine a reduction of total bile ducts in the end [8][15].
In cholangiocarcinoma (CCA), cellular growth and expansion also deserve interest as possible targets for therapy. Several molecular cascades such as the Janus kinase/signal transducer and activator of transcription, p38 MAP kinase (MAPK), Akt (AKA, protein kinase B, PKB) or fibroblast growth factor/fibroblast growth factor receptor may stimulate cell growth and impact biliary proliferation [9][10][16,17]. In this review, after a general description of cAMP signaling and its role in parenchymal and biliary cell functions, we will discuss the most recent findings regarding the relationship between the cAMP-dependent pathway and cancer of the biliary tract (e.g., cholangiocarcinoma).
2. cAMP Signaling in Hepatocytes
During starvation, glucagon stimulates cAMP/PKA/CREB signaling; conversely, glycogenolysis may be stimulated by PKA, leading to enhanced activity of glycogen phosphorylase. Evidence supporting the role of cAMP signaling in glucose and fat homeostasis has stimulated studies on liver diseases characterized by metabolic derangement, such as alcoholic liver disease (ALD) or non-alcoholic fatty liver disease (NAFLD).
Beside the effects on lipid homeostasis, cAMP may also exhibit anti-inflammatory properties in ALD. In fact, a study in isolated monocytes and rat Kupffer cells demonstrated decreased cAMP levels associated with increased release of tumor necrosis factor-α (TNF-α) after alcohol exposure [11][36]. Furthermore, cAMP has been shown to attenuate alcohol-induced oxidative damage stimulating nitric oxide synthase expression [12][37]. With regards to NAFLD, a study has shown that cAMP acts as a second messenger after glucagon-like peptide (GLP) 1 receptor stimulation, improving fatty liver accumulation, glucose homeostasis and liver serum chemistry in leptin-deficient (ob/ob) mice [13][38].
3. cAMP and Cancer
The role of cAMP and its main effector PKA in cancer has been recognized [14][68], and targeting of this signaling axis has been identified as a possible strategy for cancer therapy [15][69]. For instance, in tumor spheres obtained from a primary cell culture of medulloblastoma, cancer growth was inhibited by forskolin and enhanced by PKA inhibition [16][75]. Since PKA composed of an RI subunit was linked to increased proliferation, and enhanced expression of the RIα-type was observed in tumors, in the early 1990s, a study with an antisense oligonucleotide-repressing RIα was conducted on cancer cells [17][77]. Two trials were registered with 8-Cl-cAMP, one (Phase II, NCT00004902) on multiple myeloma and one (Phase I, NCT00021268) on colon cancer [18][82].
4. CCA and cAMP Signaling
Incidences of CCA change widely among different countries, ranging from 85 to less than 1 out of 100,000, thus reflecting the prevalence of some risk factors such as parasitic infections (Clonorchis sinensisandOpisthorchis viverrini), biliary tract disorders (PSC, hepatolithiasis, biliary cystic diseases) and also inflammatory bowel diseases [19][84]. Intrahepatic (iCCA), perihilar (pCCA) and distal (dCCA) forms are recognized with different prevalence and prognoses [20][85]. Histological classification is complex since heterogeneity is found in genetic alterations, pathogenesis and cellular origin, which are all factors contributing to a variable morphological picture [21][86]. In this perspective, since cAMP signaling plays an important role in the normal proliferative activity of cholangiocytes, it is likely that changes to this pathway may occur in biliary tract cancer.
A study examined the relationship between cAMP and secretin stimulation in CCA cell lines [22][90]. More interestingly, stimulation of CCA cell lines with secretin did not increase intracellular cAMP levels so that proliferative activities were not enhanced. This was also confirmed in an in vivo model of Mz-ChA-1 xenotransplantation in nude mice, where tumor growth was delayed by secretin treatment. The opposite effect of secretin on CCA cell growth was related to an aberrant (cancer-related) coupling of the secretin receptor with Gαirather than Gαs(Figure 23).
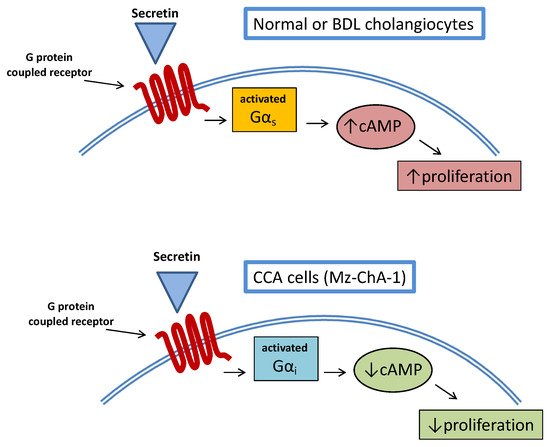
This study also had the merit of demonstrating once again the complex interplay between CCA and cAMP signaling, evidencing a mechanism similar to what was already observed, upon stimulation of the TGR5 receptor in cholangiocytes’ cilia [23][66]. A number of studies suggest a multifaceted interaction between hormones/neuropeptides and cAMP signaling with regard to CCA growth.
Other studies examined the possible role of the BA receptor TGR5 (known as a significant stimulator of cAMP/PKA signaling in cholangiocytes) [24][63] in CCA growth. These observations underscore the differential and complex effect of TGR5/cAMP signaling in cholangiocytes, according to different species and conditions. In fact, while in H69 human biliary cells (both ciliated or non-ciliated), TGR5–induced proliferation is strictly related to fluctuations of cAMP levels [24][63], in murine cholangiocytes and human CCA cell lines (EGI-1 and TFK-1). cAMP further studies, possibly on human tissue, would be needed to improve our knowledge of the TGR5/cAMP relationship in cholangiocytes and possible targeting in CCA.
