Tungsten is recognized as a critical metal due to its unique properties, economic importance, and limited sources of supply. It has wide applications where hardness, high density, high wear, and high-temperature resistance are required, such as in mining, construction, energy generation, electronics, aerospace, and defense sectors. The two primary tungsten minerals, and the only minerals of economic importance, are wolframite and scheelite. Secondary tungsten minerals are rare and generated by hydrothermal or supergene alteration rather than by atmospheric weathering. There are no reported concerns for tungsten toxicity. However, tungsten tailings and other residues may represent severe risks to human health and the environment. Tungsten metal scrap is the only secondary source for this metal but reprocessing of tungsten tailings may also become important in the future.
- tungsten resources
- mine waste
- recycling
1. Tungsten Geochemical Mobility, Toxicity and Environmental Risks
1.1. Geochemical Mobility
Koutsospyros et al. (2006) reported tungsten can be released to aquatic systems through a host of natural and anthropogenic routes from terrestrial, atmospheric and biotic environments. The natural processes of tungsten mobility include the weathering of tungsten-rich rocks and soils, dissolution of tungsten minerals, hydrothermal and volcanic activities, wet and dry atmospheric precipitation, and excretion of metabolites of tungsten enriched plants. They also reported both soluble and particulate forms of tungsten can exist in aquatic environments, but soluble tungsten is of higher environmental concern because of its higher mobility and toxicity [1]. Tungsten and its compounds usually have limited aquatic solubility and mobility. The reactions of tungsten with water play a major role in its mobility. Microbial activities can also promote tungsten mobility in solution. The iron-oxidizing bacteria and manganese-oxidizing bacteria can significantly accelerate the breakdown of tungsten minerals because these bacteria can destroy the crystalline structure of tungsten minerals and release soluble tungsten compounds [2].
Tungsten exists naturally in ocean water and sediments, surface water bodies, and groundwater in areas of hydrothermal activity [1]. Tungsten metal does not occur in nature, but tungstate anion persists and is thermodynamically stable under most environmental conditions. Tungstate anion can polymerize with itself and other oxyanions (e.g., molybdate, phosphate, and silicate) and generate a variety of polymer species. Polymerization of tungstate anion will impact the mobility of tungsten in ground or surface water due to different geochemical properties of the various polytungstates [3].
1.2. Toxicity
Research has shown that tungsten at very high concentrations and long-term contact (occupational exposure and chronic tungsten poisoning) is harmful to humans. For example, occupational exposure to tungsten may lead to pulmonary fibrosis related to hard metal lung disease, while exposure to tungsten at an environmental level concentration by breathing air, eating food, or drinking water has a low possibility to have harmful effects on humans [4]. A report from the US Center for Disease Control and Prevention (US CDC) investigated the cases of childhood leukemia and compared tungsten exposure in a control community with the community of relatively high exposure through drinking water (~45 times higher). There was no direct evidence showing that tungsten would definitively cause childhood leukemia [5], and the latest follow-up papers also supported this conclusion [6][7].
In the natural environment, tungsten is non-toxic to certain microorganisms but plays an essential role in the biology of microorganisms. Experiments even found tungsten could stimulate the growth of some species of microorganisms [8]. Tungsten also formed a variety of metal enzymes in natural biological systems, and these tungsten-containing enzymes played active roles in the anaerobic aspects of the carbon cycle. For example, tungsten could form tungstoenzymes, which occurred and prevailed in thermophilic and hyperthermophilic in the vicinity of deep-sea hydrothermal vents [9]. For plants, a study found that tungsten can be enriched by several plants [10]. For example, rice can enrich tungsten from the soil, and the enrichment factor of tungsten decreased in the following order: root, leaf, stem, and grain [11].
However, a study also reported that tungsten has potential fetus toxicity and affected the early stages of fish development [12]. In addition, Wistar rats also had a significant rise in the DNA damage and micronuclei, and a difference in biochemical levels and histopathological alterations, after 28 days of repeated oral administration of 1000 mg/kg dose of tungsten trioxide (WO
3) nanoparticles. Tungsten biodistribution was detected in all tissues in different concentrations. The highest concentration of tungsten was found in the liver, and the lowest, in the brain of treated rats. However, the overall conclusion from this experiment was that tungsten trioxide nanoparticles have little toxicity hazard even at the highest dose (1000 mg/kg bw/day dose) after 28 days of repeated oral exposure, according to the Organization for Economic Cooperation and Development (OECD) test guideline 407 (2008) [13].
1.3. Environmental Risks of Tungsten Waste
Though tungsten showed little toxicity to humans, animals, and plants, the tungsten mine wastes pose non-neglectable threats to the environment. It was reported that most of the tungsten (about 93% on average) in the soil is in the residual fraction, with low mobility and bioavailability [11]. As a result, the major pollutants released from tungsten mine waste do not necessarily relate to tungsten due to its low concentration and low mobility, but other contaminants present in tailings, such as-, Zn- and Pb-bearing sulfides, carbonates, and sulfates [14].
Acid mine drainage (AMD) generated from tungsten tailings storage facilities (TSFs) proved to be another environmental and health risk. Lianhuashan tungsten mine, one of the largest tungsten mines in southern China, is rich in polymetallic sulfide ores. The major minerals of this mine are wolframite, scheelite, arsenopyrite, pyrite, magnetite, chalcopyrite, quartz, sericite, chlorite, and feldspar. This mine was closed in 1991 and left a huge amount of untreated tailings in the TSFs. During the rainy season, AMD and weathered slag were released into the ambient environment and resulted in serious contaminations. Pollutants included Cu, Cd, Zn, Pb, Hg, and As, with As being the prominent pollutant. It also demonstrated that tungsten was not a significant contaminant element in tungsten tailings [15].
Currently, there is no environmental guideline on tungsten pollution in the United States or the European Union, neither in Australia, nor published data on the environmental effects of tungsten are inadequate [16]. Only for major tungsten substances, International Tungsten Industry Association (ITIA) published hazard classifications (
Table 1) [17].
Table 1. Hazard classifications for the major tungsten substances. Based on: [17].
| Substance | Hazard Class (EC1272/2008) | Hazard Warning |
|---|
| Ammonium Metatungstate | Acute oral toxicity 4 | Harmful if swallowed |
| Ammonium Paratungstate | Not classified | None |
| Sodium Tungstate | Acute oral toxicity 4 | Harmful if swallowed |
| Tungsten Powder (0.6–0.9 µm) | Flammable solid 1; Self-heating 2 | Flammable solid; Self-heating in large quantities; may catch fire |
| Tungsten Powder (<1.0 µm) | Flammable solid 1 | Flammable solid |
| Tungsten Powder (1.0–1.5 µm) | Flammable solid 2 | Flammable solid |
| Tungsten Powder (>1.5 µm) | Not classified | None |
| Tungsten Blue Oxide | Not classified | None |
| Tungsten Carbide | Not classified | None |
| Tungsten Disulfide | Not classified | None |
| Tungsten Trioxide | Not classified | None |
2. Potential Reprocessing Approaches for Tungsten Recovery from Tailings
2.1. A Summary of Previous Reprocessing Trials
3 [18]. Wolframite ores are of good quality, high grade, easy mining, easy selection, have convenient subsequent treatment, and less environmental hazard [19]. Gravity and magnetic separation are the most common methods for the enrichment of wolframite because it is a paramagnetic, heavy, and dense mineral. But these methods are not suitable for the recovery of ultra-fine wolframite, especially for particle sizes below 20 μm [20]. In comparison, scheelite is amenable to flotation, which is the conventional approach applied in scheelite beneficiation [21]. To date, tungsten tailings are still mainly treated as a waste rather than a resource, and only limited experiments have been done on tungsten tailings reprocessing.
3 wolframite concentrates at reasonable recovery from tungsten tailings. However, because the reprocessing of 1 t of tungsten tailings delivered only a few kilograms of tungsten concentrate, it was considered to be uneconomic [22].
Table 2
Table 2.
| Tungsten Tailings Type | Deposits | Major Tungsten Minerals | Tailings Grade, WO3% | Reprocessing Methods | Reprocessing Results | Reference |
|---|
| High-intensity magnetic separation tungsten ore slime | Rajasthan (India) | Wolframite | 2.87 and 5.30 | Polymeric dispersant with magnetic separation | Wolframite was enriched from tungsten slimes to 5.4–11% WO3 concentrates. The grade of tungsten concentrates was increased to 10% when dispersant is applied | [23][76] |
| Fine tungsten tailings | Dajishan (China) | Wolframite | 0.45 | Flotation | 30.18% WO3 concentrates with an 80% recovery rate from very fine wolframite slime | [24][77] |
| Historical mine tailings and current plant slimes tailings | Panasqueira (Portugal) | Wolframite, most of the particles below 25 µm | 0.1 | Flotation, magnetic separation, and gravity concentration | A three-stage gravity separation combined with intermediate sulfide flotation produced tungsten concentrates with 50–55% WO3 | [22][45] |
| Tin mine tailings | Potosi Mine tin processing plant (Bolivia) | Wolframite | 0.64 | Chlorination segregation, flotation, high-intensity magnetic separation, and gravity separation | 60.22% WO3 concentrate with 64.26% recovery rate; 25.04% copper concentrate with 83.19% recovery; and 40.11% tin concentrate with 65.59% recovery | [25][78] |
| Old tailing dumps | Kolar and Hutti goldfields (India) | Scheelite | 0.2 | Tabling, flotation, and magnetic separation | 65% WO3 concentrate from a feed of tungsten tailings | [26][79] |
| Old molybdenum mine tailings | Tyrnyauz processing plant (Russia) | Scheelite | 0.05 | Flotation | 54–55% WO3 concentrate with 61.91–62.08% recovery rate from wolframite-molybdenum sand tailings | [27][28][80,81] |
2.2. Gravity Separation
Gravity separation is an important approach in wolframite beneficiation. Compared with other mineral processing technologies, gravity separation has several advantages, such as high separation efficiency, low investment and operation costs, no additional chemical reagents required, and no potential pollution to the environment [29]. But conventional gravity separation is inefficient for the fine and ultrafine fractions of wolframite: it was reported to be below 45% [20]. Due to wolframite’s hard and brittle properties, the generation of over crushed wolframite is inevitable during ore grinding [30]. As a result, a large portion of fine and ultrafine wolframite may be left in tailings after beneficiation by gravity separation. Nevertheless, in the same study, enhanced gravity concentrators were successfully applied for the fine tungsten minerals beneficiation [30]. Hang and vibrate cone concentrator and Falcon concentrator are two very selective separators for fine-sized mineral particles (typically +10–75 μm) and have very high mineral upgrading ratios (typically 20 to 1). An artificial sample consisted of pure and fine minerals of scheelite, wolframite, cassiterite, fluorite, and calcite was prepared to test these two enhanced gravity concentrators for the fine tungsten minerals recovery. Most of the minerals in this sample were distributed in the -74 μm fraction, with the superfine fraction (−19 μm) accounting for more than 30%. The results showed that these two concentrators could respectively achieve 83.15% and 76.38% tungsten recovery rates. However, it was also found that these separators were still not efficient in the recovery of ultrafine (−10 μm) wolframite particles [29].
2.3. Magnetic Separation
In wolframite beneficiation, magnetic separation is usually operated in a high-intensity magnetic separation system for ideal wolframite recovery. In the conventional magnetic separation process, wolframite particle size plays a prominent role, similarly to gravity separation. With a decrease in the wolframite particle size, magnetic forces acting on the wolframite particle would drop quickly and cannot resist a hydrodynamic drag. As a result, the fine fraction of wolframite is lost to tailings. Furthermore, feeding flow and washing water can also wash away ultrafine wolframite from the separation plates in the magnetic field [31].
Figure 1 shows that the recovery of wolframite through magnetic separation varies under different particle sizes and magnetic intensities. For the particle sizes above 10 μm, the maximum recovery of wolframite could reach approximately 90% with an increase in magnetic intensity to 1.3 Tesla (1.3 T). However, for wolframite particles below 10 μm, the maximum recovery could only achieve approximately 60%, even with the highest 1.5 T magnetic intensity [31].
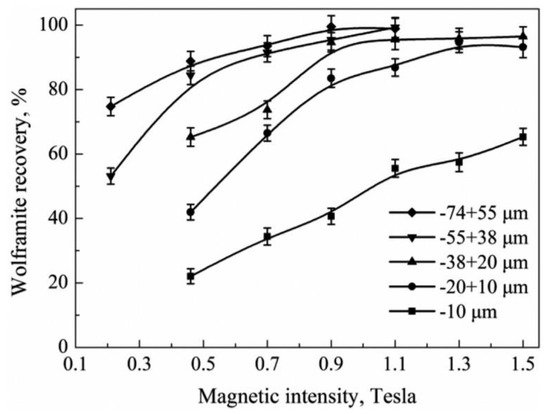
Figure 1. Recovery of different size fractions of wolframite as a function of magnetic intensity [31].
Wet high-intensity magnetic separation (WHIMS) has been used as an effective enhanced approach to separate minerals of low magnetic susceptibility from tailings [32]. A WHIMS modeling on tungsten tailings predicted good wolframite recovery rates: approximately 80% (90%) for new tailings and 65% (80%) for old tailings at 0.9 T (1.6 T) magnetic intensity [33]. Another study also found that WHIMS can successfully recover the fine fraction of wolframite from tungsten ore slimes and reach 90% recovery at 1.15 T magnetic intensity [23].
2.4. Flotation
Flotation is a physicochemical separation process for minerals, which usually utilizes various minerals’ surface properties to separate valuable minerals and unwanted gangue minerals. Selective flocculants, dispersants, depressants, and flotation collectors are usually used in flotation. Major influencing factors for flotation are mineral surface wettability, surface lattice ion dissolution, surface electrical properties, and solution chemical properties [34]. For scheelite ores, especially occurring in skarns, fluorite, apatite, and calcite constitute common gangue minerals, flotation is currently the major processing method [35]. Frequently used scheelite flotation depressants are sodium silicate, phosphates, and fluorosilicates [19].
For wolframite, because of its low floatability, selective flotation was hardly used for its beneficiation at an industrial scale. However, for ultrafine wolframite, due to its poor recovery by conventional gravity separation and magnetic separation, flotation was successfully applied. For example, froth flotation, shear flocculation, and spherical agglomeration with highly selective reagents, such as alkyl hydroxamates, phosphonic acid derivatives, and alkylated nitroso-napthols, can significantly enrich fine and ultrafine wolframite from tungsten ore slimes [36]. It was also reported that benzohydroxamic acid (BHA) and sodium oleate (NaOl) as flotation collectors could efficiently improve the fine wolframite collecting capability [37]. While adding lead ions (Pb
2+) into flotation collectors could improve the adsorption of BHA onto the wolframite surface in the pH range from 4 to 10.5, which can significantly increase the recovery of fine wolframite in flotation [38]. More recent studies found that a novel surfactant N-(6-(hydroxyamino)-6-oxohexyl) octanamide (NHOO) is a more efficient collector in wolframite flotation [39].
3
3 fine wolframite slime [30]. However, though flotation could enrich fine and ultra-fine wolframite and reprocess tungsten tailings, the flotation beneficiation reagents left in the new tailings can result in new contamination to the environment, especially some arsenic (As)-bearing reagents (e.g., arsonic acid).
2.5. Chemical Leaching
Chemical leaching for minerals beneficiation is heap leaching. It is a relatively low-cost processing method, widely used for metal extraction from low-grade ores, including copper (Cu), gold (Au), silver (Ag), and uranium (U) [40]. Furthermore, it was also used in soil remediation, reprocessing agglomerated flotation tailings, and for the treatment of coarse rejects from semi-autogenous grinding circuits [41]. This method usually involves acids, alkalis, and cyanide to mobilize and collect valuable metals in solution. However, the main drawbacks of chemical heap leaching are that it can be slow and inefficient and may result in potential risks to the environment [42].
Although chemical heap leaching could be used to reprocess tailings and residue materials [42], it is unlikely to reprocess tungsten tailings. Scheelite is hard to decompose by acids at normal temperature because the generation of the solid-colloidal layer of tungstic acid (H
2
4) on the scheelite surface would stop its further decomposition [43]. While decomposing scheelite with sodium hydroxide (NaOH) or sodium carbonate (Na
2
3), digestion would require the reaction temperature above 180 °C in an autoclave [44]. It was also reported that scheelite could not be decomposed by NaOH under commercial conditions [45]. Similarly, scheelite leaching with hydrochloric acid (HCl) for tungstic acid would require a reaction temperature above 125 °C [46]. It was almost the same condition needed for wolframite decomposition. Caustic digestion is a conventional method employed to decompose wolframite to produce soluble tungstate (WO
42−) in the industry. However, it may require higher temperature, pressure, and some other conditions [47][48]. Therefore, it is not feasible for reprocessing of tungsten tailings through chemical heap leaching under natural conditions.
2.6. Bioleaching
Over the past decades, bioleaching has quickly developed and is used to recover metals from ores in the mining industry. So far, bioleaching has been applied to extract zinc (Zn), cobalt (Co), copper (Cu), nickel (Ni), lead (Pb), gold (Au), and arsenic (As) from minerals in the industry [49][50]. For example, bioleaching of copper can achieve a higher than 90% recovery rate, and each year approximately 20% of global copper is produced through bioleaching [51]. Compared with conventional mineral processing methods, bioleaching is low-cost, highly safe, simple to operate, and environmentally friendly [52].
Currently, bioleaching is more and more frequently applied to extract, recover and remove heavy metals from solid waste as well, including mine tailings and sediments [53][54]. In most cases, acidophilic chemolithotrophic microorganisms are the major microbes for bioleaching. Biological oxidation and complexation reactions are the ways for bioleaching to mobilize metal cations from insoluble minerals [55]. However, bioleaching is sensitive to several conditions, such as solids concentration, temperature, oxygen, pH, redox potential, bacterial strain, and cell concentration. These factors play important roles in the optimization of the bioleaching process [56]. Besides single strain microbe of metal sulfide bioleaching, mixed microbe cultures can be a more efficient way to decompose minerals [57]. Mixed cultures can use elemental sulfur (S
0
2+
2) or use organic carbon as a carbon source. Usually, it is a much more stable and effective mineral bioleaching consortium. Different bacterial strains in the consortium can also cooperate to respond better to environmental changes during bioleaching [58]. The feasibility of bioleaching for mine tailing reprocessing can be supported by its successful application in the mining industry worldwide.
The growing demand for metals has already led to the re-assessment of old tailings as a potential resource. A few studies have proved that valuable or toxic elements in tailings can be recovered or removed by bioleaching in appropriate ways. A study has demonstrated a successful application of bioleaching to remove heavy metals from low-grade Zn-Pb mine tailings [59]. The experiments also showed that bioleaching is quite effective in recovering Zn and In from old Zn-Pb tailings, with respective recovery rates of up to 100% and 80% [60]. For tungsten minerals, the latest research demonstrated that some microbes (the extreme thermoacidophile
Metallosphaera sedula) can grow on and directly extract tungsten from scheelite, which proposed a new approach for tungsten tailings reprocessing [61]. One successful bioleaching experiment on tungsten tailings was to remove As and manganese (Mn) from tungsten tailings. When mixed cultures of
Acidithiobacillus ferrooxidans
Acidithiobacillus thiooxidans were applied, the recovery of As could reach 96.7%, and the recovery of Mn almost 100% [62][63].
