1. The Photic Zone: Where Light Fuels Life in the Ocean
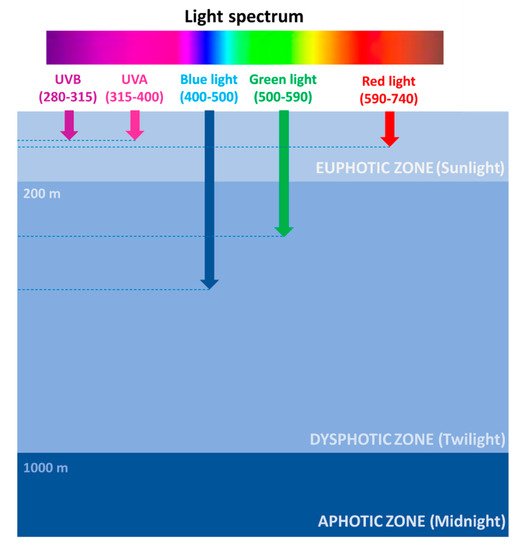
The photic zone is the uppermost layer of the sea, distinguishable in two main areas: the euphotic (or sunlight) zone, approximately 200 m deep, where sunlight allows phytoplankton to accomplish photosynthesis, and the dysphotic (or twilight) zone (200–1000 m deep), where sunlight rapidly decreases with depth and where photosynthesis cannot occur (Figure 1).
Figure 1. Light spectrum penetration at sea.
However, the depth of the euphotic zone depends on the transparency of the water. If the water is very clear, light can penetrate deeper, while if the water is murky, the depth of the euphotic zone is reduced to only 50 feet (15 m). Ninety-five percent of photosynthesis in the ocean occurs in this zone, where the photosynthesis rate exceeds the respiration rate. Therefore, the high oxygen concentration fuels the life of 90% of marine organisms, including phytoplankton, such as dinoflagellates, diatoms, and cyanobacteria; zooplankton, such as copepods; and nekton, such as fish, squids, and crabs.
In the ocean, exposure to sunlight is extremely variable along the water column, both in terms of photon flux density and spectral composition (
Figure 1). Several factors are responsible for light intensity and spectrum variability at sea, including the geographical location, the day hours/season, the weather conditions, and the occurrence of organic matter. Red wavelengths (589–656 nm) are absorbed in the euphotic zone and are rapidly attenuated in the first water layers, thus reaching approximately 100 m in depth. Green wavelengths (479–579 nm) can penetrate until a depth of approximately 300 m and are used by algae for photosynthesis. Blue wavelengths (422–496 nm) can penetrate farther, being the only ones to reach the deep ocean, approximately 500 m in depth, and are mostly responsible for the color of the oceans
[1][2][7,8]. The prevalence of blue/green light at sea has prompted marine organisms to sense and respond prevalently to these wavelengths, even though red/far-red sensing systems have also been described in diatoms
[3][9].
2. Photo-Damage/-Protection Mechanisms in Marine Organisms
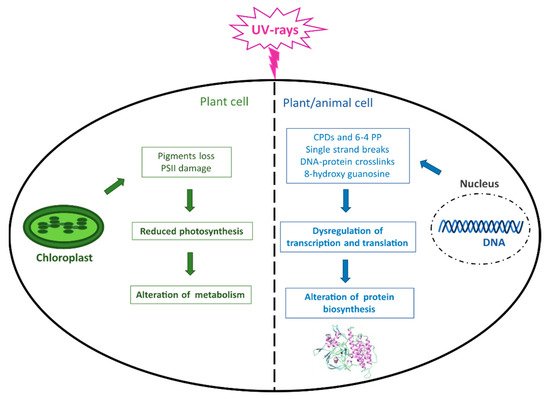
Although light is essential for life on Earth and in the oceans, photosynthetic organisms can be harmed by an excess of photons reaching the photosynthetic apparatus, whose damage can result in a decrease in photosynthesis and metabolism (
Figure 2). Moreover, the major macromolecules, including proteins, lipids, and especially DNA, can be damaged by both direct UVR and consequent ROS production, which ultimately leads to altered protein biosynthesis and crucial cellular functions not only in plant but also in animal organisms (
Figure 2).
Figure 2. UVR-induced damage in living organisms. The figure summarizes the main effects caused by UVR occurring at the DNA level in both plant and animal cells and in chloroplasts in photosynthetic cells. CPDs = cyclobutane pyrimidine dimers; 6–4 PP = pyrimidine (6–4) pyrimidone photoproducts; PSII = Photosystem II.
Such an environmental constraint has forced them to evolve a complex system of photoprotective mechanisms and antioxidant enzymes and molecules, in order to counteract light-dependent stress. For instance, the first defense mechanism used by algae against high light is the dissipation of excess light into heat through non-photochemical quenching (NPQ) of chlorophyll fluorescence. This mechanism allows algae to prevent photodamage
[4][24]. In particular, the increased proton flux leads to acidification of the thylakoid lumen inside chloroplasts, with consequent activation of the xanthophyll cycle, a key player in the NPQ process
[5][25]. In addition to these short-term responses, cells have adopted a variety of long-term responses to counteract light stress, including several integrated signaling pathways and associated changes in gene expression. In particular, variations in the state of chloroplasts are sensed by the nucleus through a “retrograde signaling” in which the tetrapyrroles of chlorophyll seem to play a central role
[6][26].
However, despite such protective mechanisms, high light exposure can ultimately lead to the formation of ROS. Algae possess a complex enzymatic and non-enzymatic defense system to prevent photo-oxidative damage to cellular components. Enzymatic antioxidant protection includes three types of superoxide dismutases possessing different metal cofactors (CuZn-SOD, Fe-SOD, Mn-SOD), catalase, ascorbate peroxidase, glutathione peroxidase, while the non-enzymatic defense system includes ascorbic acid, glutathione, tocopherols, carotenoids, polyphenols, phycobilin proteins, dimethylsulfide/dimethylsulfoxide, sulfated polysaccharides
[7][27], and sulfur-containing histidines such as ovothiols
[8][9][28,29].
3. UV Filter Properties
Typically, a sunscreen is conceived as a formulation containing UV chemical filters, which can be either inorganic, such as zinc oxide (ZnO) and titanium dioxide (TiO
2), or organic, such as cinnamates and benzophenones
[10][11][50,51]. Conceptually, the absorption of the UVR photons’ energy causes a photochemical excitation of the filter molecule to a higher energy excited state. As the excited molecule returns to its ground state, the excess energy is partially dissipated as heat. In some cases, the absorbed energy may be transferred to a receptor molecule, or emitted in the visible region and perceived as fluorescence, or it may be emitted in the longwave UVR (380–450 nm), which may cause a photochemical reaction such as cis-trans or keto-enol isomerizations in a fraction of the filter molecules. These photochemical reactions, in some cases, may give rise to instability in the filter molecule, with chemical bond cleavage and formation of photodegradation products. This is a highly undesirable property for a UV filter since the loss in absorption efficacy defeats the purpose of a UV filter for sunscreen use. An efficient UV filter should be able to return to its ground state in its original form such that it may be available to absorb another photon. This guarantees photoprotection throughout the entire duration of UV exposure
[12][52].
Organic UV filters used in sunscreens are powerful photochemicals whose behavior is closely related to their molecular structure. They typically contain a chromophore, which is usually an aromatic molecule conjugated to carbonyl groups. In general, an increased number of conjugated double bonds and resonance structures stabilizes the excited state, shifting the absorption spectrum of the compound to longer wavelengths. Instead, inorganic filters such as ZnO and TiO
2, commonly used in sunscreen formulations, besides absorbing UV light, also possess scattering and reflecting properties. They are generally considered more natural and benign than organic UV filters, despite the fact that they contain a wide array of coatings, additives, predispersants, and dispersion enhancers. Furthermore, because of their particulate nature, which causes the so-called “whitening” phenomenon upon application, they are nowadays generally micronized to improve their cosmetic appearance
[13][53].
There is a growing body of evidence suggesting that the synthetic UVR filters such as octocrylene, benzophenone-4, ethyl 4-aminobenzoate, 3-benzylidene camphor, and TiO
2 nanoparticles may cause damage to the marine environment, due to their widespread use on sea resorts. Several negative effects include the bioaccumulation of filters in many different species, hormonal changes and endocrine disruption in fish, abnormal development in sea urchins, and the bleaching of corals
[14][15][16][17][54,55,56,57].
4. Mycosporine-Like Amino Acids (MAAs)
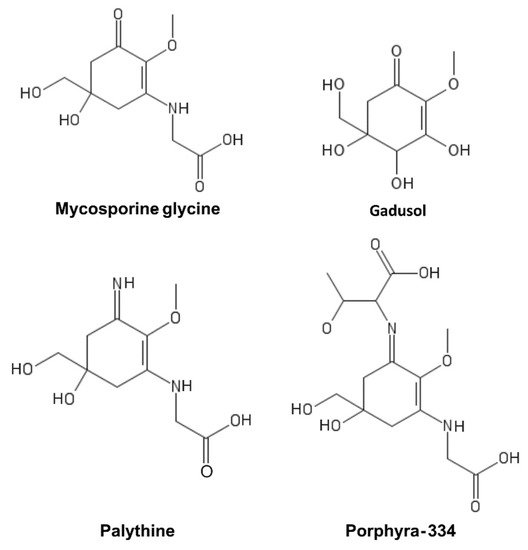
MAAs are a heterogeneous group of hydrophilic molecules (<400 Da). Their structure consists of a cyclohexanone or cyclohexenimine ring system conjugated to an amino acid or its imino alcohol
[18][59]. Representative MAAs are reported in
Figure 4.
Figure 4. Mycosporine-like amino acids (MAAs).
They absorb in the 310–362 nm range with a high molar extinction coefficient and are produced by organisms that are particularly exposed to UV light, such as cyanobacteria
[19][60], micro- and macroalgae
[20][21][61,62], dinoflagellates
[22][63], and fungi
[23][64]. Their biosynthesis is mainly ascribed to the shikimate pathway, although the pentose-phosphate pathway has also been suggested to be involved in their biosynthesis
[24][25][65,66]. Aromatic amino acids and MAAs in marine organisms have been generally hypothesized to be of dietary origin since most metazoans lack genes coding for the enzymes of the shikimic acid pathway
[23][26][27][64,67,68]. However, previous studies have suggested that cnidarians, including nonsymbiotic corals and anemones, are capable of synthesizing these essential amino acids
[27][28][29][30][68,69,70,71]. Starcevic and colleagues
[29][70] provided evidence for the horizontal transfer of both bacterial and dinoflagellate ancestral genes of the shikimic acid pathway into the genome of
Nematostella. Moreover, besides corals, the endogenous synthesis of gadusol, the main MAAs precursor, has been discovered to occur in fish through an alternative pathway, which is also present in amphibians, reptiles, and birds
[31][32][72,73].
MAAs play an important sunscreen role in producer organisms due to their properties, which include strong UVA absorbance, photostability, and widespread distribution in cell cytoplasm
[27][68]. In addition, MAAs possess high stability under different pH and temperature values, representing a key advantage for their use as UV filters
[33][74]. Furthermore, MAAs also function as strong antioxidants and additional roles have been suggested in cellular osmotic regulation during salt stress or as nitrogen reservoirs during nitrogen limitation
[22][34][63,75]. Several studies have reported the ability of MAAs to protect against DNA damage induced directly by UVR and indirectly by ROS production triggered either chemically or by UVA radiation
[35][36][37][76,77,78]. MAAs have also shown promising antioxidant, anti-inflammatory, and anti-aging activities in in vitro studies on human cells. In particular, their anti-aging properties were shown to be partially mediated by a direct inhibitory activity on protein glycation and collagenase enzymes
[38][79]. To date, only a few products that include MAAs in their formulations, such as Helioguard
®365 and Helionori
®, have been released
[39][40][80,81]. Among them, palythine, extracted from the red alga
Chondrus yendoi has recently been incorporated as an active ingredient in the skin care product line Aethic
®, and launched on the market in 2021 as Dermagie. These algae can be harvested year-round and can protect human skin cells from UVA and UVB damage under artificial sunlight as intense as at noon during summer in Arizona
[37][78]. Nevertheless, further studies are necessary to fully assess the efficacy of MAAs for application in topical formulations
[40][41][81,82].
5. Scytonemin
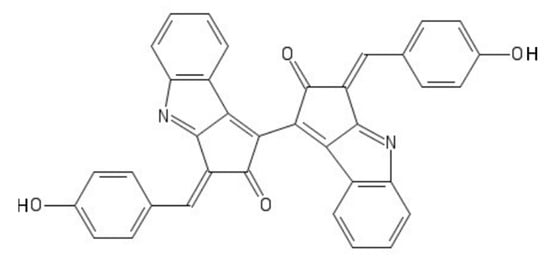
Scytonemin is a hydrophobic dimeric molecule composed of an indolic and phenolic subunit, linked by a carbon–carbon bond
[42][83]. Its complex ring structure (
Figure 5) allows a wide absorption range not only in the UVB but also in the UVA range, reaching a maximum of 370 nm
[42][83].
It is produced by some species of cyanobacteria, such as
Scytonema sp., having the ability to produce extracellular polysaccharides (EPS) and living in harsh environments typically characterized by desiccation periods, high temperatures, and nutrient limitation
[43][44][45][46][84,85,86,87]. Scytonemin-producing cyanobacteria secrete this molecule in the extracellular matrix, where it acts as a physical barrier against UVA and as an antioxidant molecule against ROS produced following UVA exposure or several other abiotic stressors
[45][47][48][86,88,89]. Scytonemin biosynthesis includes the involvement of enzymes from the shikimate pathway, tyrosine and tryptophan biosynthesis, and two additional specific enzymes called ScyB and ScyA
[49][90].
The high extinction coefficient (ε = 250 Lg
−1cm
−1 at 384 nm) and the exceptionally high photostability make scytonemin an efficient photoprotective chemical
[50][91]. The UV-protective abilities of scytonemin extracted from cyanobacterial biofilm have been demonstrated in UV-irradiated
E. coli cells
[51][92].
In addition, scytonemin is also endowed with other biological activities, acting as an antioxidant
[45][86], anti-inflammatory
[52][93], and antiproliferative agent
[53][54][55][94,95,96], and as a calcium-channel blocker
[56][97]. Moreover, due to its presence in organisms able to survive in extreme environments, it has been proposed as a biomarker for the identification of life on extraterrestrial planets
[57][58][98,99].
6. Polyphenols
Polyphenols are a large class of secondary metabolites characterized by the presence of several phenol rings. Among them, phenolic acids and flavonoids are the most abundant
[59][100]. They are mainly found in plants, including fruits, vegetables, tea, and other plant-derived beverages. Due to their pleiotropic properties, they are endowed with numerous beneficial properties for human health, ranging from the prevention of cardiovascular diseases, metabolic syndrome, and neurodegenerative disorders, to anti-aging, anticancer, and antimicrobial activities
[60][61][62][101,102,103]. They have also recently been proposed as an alternative co-adjuvant therapy against coronavirus infections, or in combination with classical antiviral drugs
[63][104].
Besides terrestrial sources, marine organisms are also able to produce polyphenols, especially flavonoids. Most marine flavonoids have been found in seagrasses and halophytes but they have been identified also in mangroves, bacteria, algae, fungi, and corals. Some of these molecules present unique substituents compared to their terrestrial counterparts, such as the occurrence of sulphate, methyl, chlorine, amino acids, and aminodeoxy sugar groups
[64][105]. Marine polyphenols have been shown to have the classical pharmacological activities ascribed to those of terrestrial origin, such as antioxidant, antidiabetic, anticoagulant, antimicrobial, and anticancer properties, and, in addition, they possess antifouling and antifeedant activities
[64][105].
Photoprotective activities have also been described for polyphenols, whose action is either direct, due to their UV absorption ability, or indirect, thanks to their antioxidant/scavenging function and consequent regulation of different signaling pathways
[65][66][106,107]. For instance, polyphenols extracted from brown algae have been reported to exert protection against UVB-induced damage in human cells and in an in vivo zebrafish model
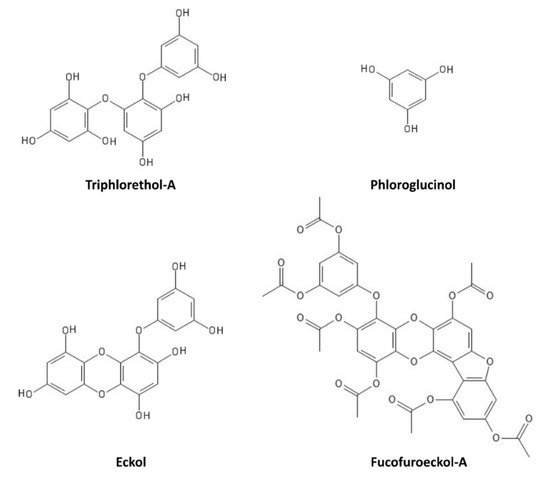
[67][68][69][70][108,109,110,111]. In particular, triphlorethol-A (
Figure 6) protects human keratinocytes (HaCat) against UVB-induced damage, by exerting a UV-filtering and antioxidant function, as well as by inhibiting the caspase pathway
[67][108]. Phloroglucinol (
Figure 6) also showed UVB protection properties in HaCat cells, through the inhibition of key molecular mediators of UVB-induced photoaging, including MMP-1 (matrix metalloproteinase-1) activity, Ca
2+ levels increase, MAPK (mitogen-activated protein kinase) phosphorylation, c-Fos and phospho c-Jun expression, and AP-1 (activator protein-1) binding to the MMP-1 promoter
[68][109]. Other polyphenols, such as the phlorotannin eckol (
Figure 6), protect human keratinocytes against UVB-induced oxidative stress by scavenging ROS and reducing apoptosis by inhibiting mitochondrial membrane disruption, thus ameliorating injury to cellular components
[69][110]. Dieckol (composed of two molecules of eckol) showed a particularly strong UVB-protective ability both in HaCat cells and in live zebrafish, where it reduced ROS, NO (nitric oxide), and cell death
[70][111]. Another derivative of eckol, fucofuroeckol-A (
Figure 6), showed photoprotective properties since it reduced the UVB-induced allergic reaction in RBL-2H3 mast cells, leading to the suppression of mast cell degranulation and decreasing histamine release and Ca
2+ levels
[71][112]. Because of these photoprotective properties, polyphenols could hence be repurposed for cosmetics development in the sunscreen sector
[72][113].
Figure 6. Marine polyphenols with UVR-protective properties.
7. Carotenoids
Carotenoids are a broad class of tetraterpenoids formed by 5-carbon isoprene units, polymerized to generate 40-carbon structures containing up to 15 conjugated double bonds. They include two major categories, carotenes and xanthophylls, with the latter possessing oxygen atoms in their structures. Some carotenoids consist of 6-carbon rings at one or both ends of the molecule; in the case of the xanthophylls, they possess oxo, hydroxy, or epoxy substitutions
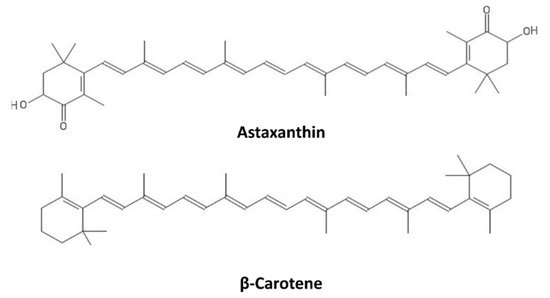
[73][114]. The structures of β-carotene and astaxanthin, as representatives of carotenes and xanthophylls, respectively, are shown in
Figure 7.
Carotenoids absorb in the blue-green region of the light spectrum (450–550 nm), and in photosynthetic organisms, they act as both light-harvesting molecules and as photoprotective agents through their ROS scavenging activity, thus contributing to most of the NPQ
[74][115]. While photosynthetic organisms, including unicellular cyanobacteria and microalgae or multicellular algae and plants, are natural producers of carotenoids, marine animals can only acquire them through the diet or symbiotic relationships, such that they can also benefit from the valuable properties of carotenoids, including photoprotective action
[75][116]. Carotenoids are mainly known as precursors of vitamin A biosynthesis in humans; however, they are endowed with multiple beneficial activities against neurodegenerative diseases, cancer, and cardiovascular pathologies
[76][117]. Several studies have reported promising photoprotective activities of carotenoids against UV light-induced erythema
[77][118]. In this process, the singlet oxygen (
1O
2) quenching activity is crucial, but they also activate different signaling pathways. β-Carotene, for instance, induces gene expression changes in human keratinocytes, resulting in decreased extracellular matrix degradation and increased cell differentiation. Both these effects were enhanced following keratinocyte irradiation with UVA light
[78][119]. These mechanisms of action highlight the potentialities of β-carotene as a protective agent against photoaging and skin diseases, such as skin cancer and psoriasis. Two marine carotenoids, astaxanthin and fucoxanthin, also revealed promising anti-inflammatory and immunostimulant activity in addition to their protective properties against DNA damage and oxidative stress
[79][120]. Astaxanthin, especially, has been tested in in vivo studies and clinical trials. Indeed, the application of a liposomal preparation of astaxanthin on the dorsal skin of Hos:HR-1 hairless mice prevented wrinkle formation induced by UV exposure
[80][121] and its effects were confirmed by additional studies on animal models
[81][82][122,123]. Moreover, astaxanthin supplementation in humans significantly increased their minimal erythema dose
[83][124], defined as “the smallest UV dose that produces perceptible redness of the skin (erythema) with clearly defined borders at 16 to 24 h after UV exposure”, and different other clinical trials highlighted the prevention and curing abilities of astaxanthin against photoaging
[84][85][86][87][125,126,127,128].
8. Sulfated Polysaccharides
Sulfated polysaccharides (SPs) consist of more than 10 monosaccharides, possessing sulfate groups on the hydroxyls of sugar units. They are present in several natural sources, such as plants, or can be obtained by chemical substitution of non-sulfated polysaccharides
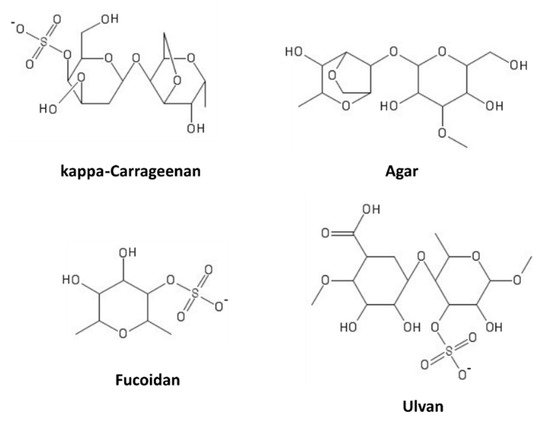
[88][129]. Marine macroalgae (seaweeds) are rich sources of these compounds. Based on their sugar composition, stereochemistry, and glycosidic linkages, algal SPs can be distinguished in sulfated galactans (carrageenans and agarans, produced by red algae), ulvans (in green algae), and fucans (in brown algae)
[89][130]. Some representatives of these classes are shown in
Figure 8.
Figure 8. Marine sulfated polysaccharides.
Some of them play structural roles in the cell wall composition, while others represent a sort of energy storage. Little is known about other physiological functions of SPs
[90][91][131,132]; for example, they have been suggested to confer resistance against desiccation and osmotic stress in marine plants
[92][133]. In addition, SPs have also been found in marine sponges, where they are presumably involved in species-specific cell aggregation and structural integrity, resembling the structural function of glycosaminoglycans in the connective tissues of mammals
[93][134]. For their gelling properties, SPs are used in drug delivery systems, while, for their numerous bioactivities, including their ability to modulate metabolism and the immune system, and their anticancer, antimicrobial, and anticoagulant properties, they possess many applications in the cosmetic, nutraceutical, and pharmaceutical industries
[94][95][135,136].
SPs do not absorb in the UV region; nevertheless, they showed strong UV-protective activities in in vitro and in vivo studies
[96][97][98][137,138,139]. The protective effects of SPs against UVB-induced skin damage were tested in vitro in human dermal fibroblasts. SPs significantly reduced intracellular ROS levels and improved the viability of UVB-irradiated cells in a dose-dependent manner. Furthermore, they significantly inhibited intracellular collagenase and elastase activities, prevented collagen synthesis, and reduced MMP expression by the NF-κB and MAPK signaling pathways
[97][138]. In addition, in vivo tests also demonstrated that SPs significantly reduce intracellular ROS levels, cell death, NO production, and lipid peroxidation levels in UVB-irradiated zebrafish in a dose-dependent manner
[98][139]. These results pointed to SPs as potential ingredients in the cosmeceutical industry.