Age-related macular degeneration (AMD), the main cause of vision loss in the elderly, is associated with oxidation in the retina cells promoting telomere attrition. Activation of telomerase was reported to improve macular functions in AMD patients. The catalytic subunit of human telomerase (hTERT) may directly interact with proteins important for senescence, DNA damage response, and autophagy, which are impaired in AMD. hTERT interaction with mTORC1 (mTOR (mechanistic target of rapamycin) complex 1) and PINK1 (PTEN-induced kinase 1) activates macroautophagy and mitophagy, respectively, and removes cellular debris accumulated over AMD progression. Ectopic expression of telomerase in retinal pigment epithelium (RPE) cells lengthened telomeres, reduced senescence, and extended their lifespan.
- age-related macular degeneration
- AMD
- telomerase
- hTERT
- autophagy
- PGC-1α
- peroxisome proliferator-activated receptor gamma coactivator 1 alpha
- mTORC1
- senescence
- DNA damage response
1. Introduction
2. Age-Related Macular Degeneration
The retina is the most energy-demanding tissue in the human body due to the activity of photoreceptors that convert a light pulse into electric signal. Age-related macular degeneration (AMD) is an eye disease affecting the macula, a small structure in the center of the retina containing the fovea, responsible for sharp and color vision. AMD is a serious physical and mental problem for individuals and a high burden for societies [8]. Clinically, AMD is classified into dry (atrophic) and wet (neovascular) forms (Figure 1).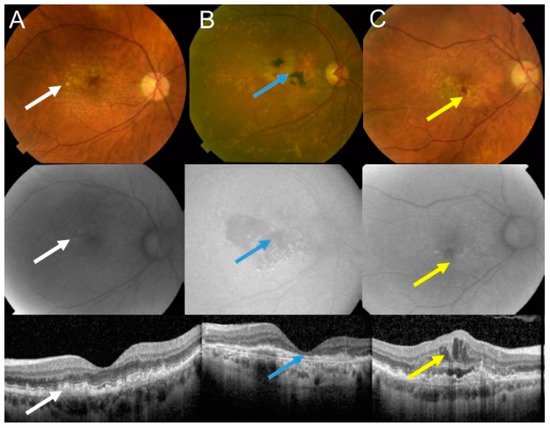
The pathogenesis of AMD is not completely known and many factors, both genetic/epigenetic and environmental/lifestyle-based, are involved. Oxidative stress is associated with AMD, but it is not exactly known what the source of such stress is and, in some cases, whether it belongs to the causes or consequences of the disease. Furthermore, oxidative stress can be linked with several putative or established AMD risk factors, including aging, smoking, blue light, obesity, and a diet rich in fat and carbohydrates [9][10][11]. The retina belongs to the most metabolically active tissues in humans with the highest oxygen consumption, resulting in the production of reactive oxygen species (ROS) as byproducts of retinal metabolism [12]. Likewise, aging, as per its definition as the main AMD risk factor, is associated with oxidative stress [13][14]. Also, mitochondria, the main source of energy and ROS production, may be central for AMD pathogenesis [15].
3. Telomeres and Telomerase in AMD
Telomeres are DNA–protein complexes at the ends of linear chromosomes in eukaryotes. They protect chromosomes against cellular exonucleases and prevent their recognition as a DNA double-strand break by DNA damage response (DDR) and chromosomal fusion. Telomerase is an RNA-dependent DNA polymerase, which does not require an exogenous template to synthesize DNA.
Drigeard Desgarnier et al. analyzed the length of telomeres in different structures of the human eye [16]. They found that neural retina had the longest telomeres, whereas the cornea had the shortest. The length of telomeres in RPE cells was about four times shorter than in the neural retina. These authors did not observed either age-dependent telomere attrition in the retina or any difference in the telomere length between the macula and the rest of the retina.
High content of guanine in telomeric DNA may have at least two consequences. The first is the natural tendency of single-stranded, guanine-rich DNA to fold onto itself to form four-stranded structures due to the ability of guanine to form highly stable hydrogen bonds with other guanines—guanine quartets [17]. This property is important in the context of telomerase action, as four-stranded DNA is not a substrate for that enzyme. The second consequence follows from the fact that guanine has the highest number of oxidation-sensitive sites among all DNA bases [18]. Therefore, telomeres may be remarkably prone to oxidative stress, a common factor of AMD pathogenesis.
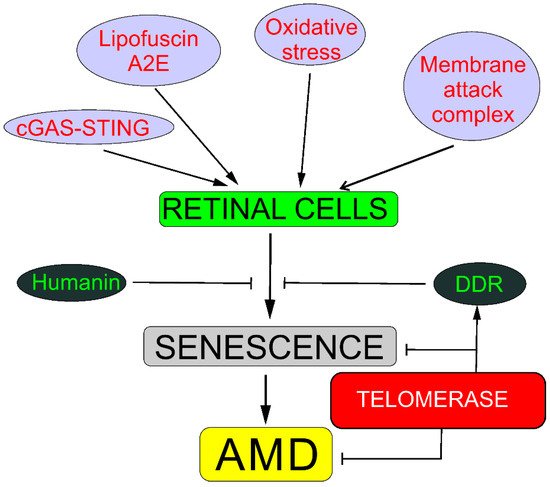
4. Senescence, DNA Damage Response, and Autophagy May Underline the Involvement of Telomerase in AMD
Reactive oxygen species produced in oxidative stress may damage DNA and other biomolecules, including those important for DDR [21]. Therefore, impaired DDR may contribute to AMD pathogenesis. Furthermore, oxidative stress and ROS induce stress-induced senescence, different from replicative senescence, but with an even worse outcome [2]. As AMD belongs to the category of proteinopathies, disorders in which protein debris are formed, impaired autophagy is associated with AMD, but the mechanisms underlying this association are still incompletely known [22][23]. Telomerase is, per its definition, engaged in preventing replicative senescence and is reported to be involved in DDR and autophagy.
4.1. Senescence
There is a direct association between senescence and telomerase—telomerase prevents senescence in proliferating cells, extending their telomeres and protecting the cells against deletion in essential genes. Moreover, telomerase may extend telomeres that are shortened due to oxidative stress or any other stress resulting in telomere-associated DNA or protein damage.
4.2. DNA Damage Response
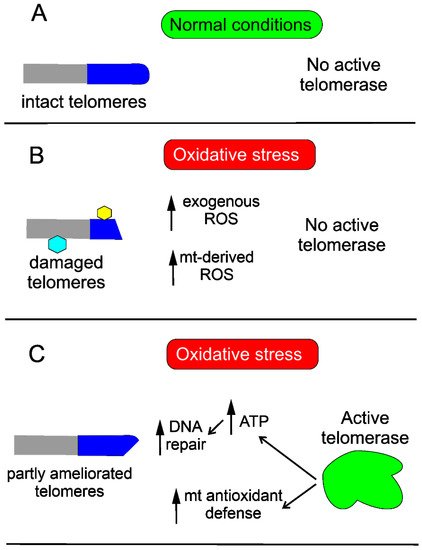
Oxidative stress is associated with overproduction of ROS, which may damage cellular macromolecules, including DNA. Oxidative stress damages telomeres, as their DNA is guanine-rich, and this is why they may be more susceptible to oxidative DNA damage than the “average” DNA in the rest of chromosomes.
Telomerase may protect the cell against DNA damage through various mechanisms. Saretzki’s lab showed that overexpression of hTERT in human fibroblasts resulted in a decrease in mtDNA damage induced by oxidative stress [24]. To search for the mechanism underlying the observed changes, they showed that telomerase did not seem to increase the repair of mtDNA damaged by oxidative stress but induced an mitochondrial antioxidant defense mechanism [25]. Altogether, these results show that mitochondrial telomerase may protect nuclear DNA (nDNA) from oxidative stress-induced damage by decreasing mitochondrially produced ROS, which was directly shown for cancer cells [26]. However, it was demonstrated that ectopic expression of hTERT in human primary fibroblasts improved the kinetics of nDNA repair, likely due to an increase in the ATP level [27] (Figure 3).
4.3. Autophagy
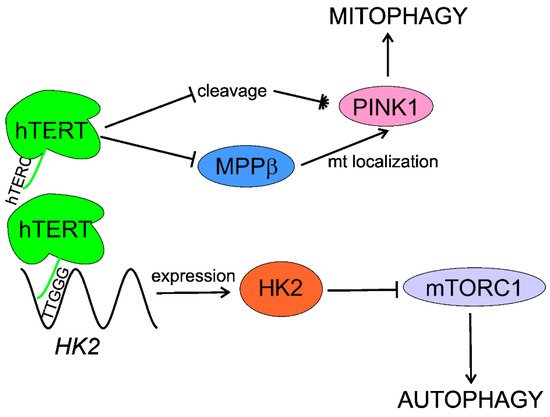
In summary, telomerase activates/stimulates autophagy through mTORC1 inhibition that may delay aging and age-related pathologies, including AMD. Telomerase may specifically activate mitophagy by regulation of the PINK1 protein. Although several independent studies show autophagy activation by telomerase, the exact mechanism of this activation is unknown and HK2 can lie between telomerase and autophagy (Figure 4).
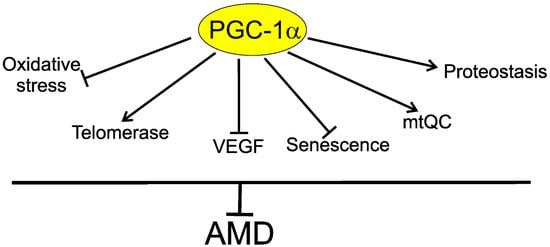
5. PGC-1α May Link Telomerase with AMD
References
- Casagrande, S.; Hau, M. Telomere attrition: Metabolic regulation and signalling function? Biol. Lett. 2019, 15, 20180885.
- Blasiak, J. Senescence in the pathogenesis of age-related macular degeneration. Cell. Mol. Life Sci. 2020, 77, 789–805.
- Victorelli, S.; Passos, J.F. Telomeres and Cell Senescence—Size Matters Not. EBioMedicine 2017, 21, 14–20.
- Collins, K.; Mitchell, J.R. Telomerase in the human organism. Oncogene 2002, 21, 564–579.
- Ghosh, A.; Saginc, G.; Leow, S.C.; Khattar, E.; Shin, E.M.; Yan, T.D.; Wong, M.; Zhang, Z.; Li, G.; Sung, W.K.; et al. Telomerase directly regulates NF-κB-dependent transcription. Nat. Cell Biol. 2012, 14, 1270–1281.
- Koh, C.M.; Khattar, E.; Leow, S.C.; Liu, C.Y.; Muller, J.; Ang, W.X.; Li, Y.; Franzoso, G.; Li, S.; Guccione, E.; et al. Telomerase regulates MYC-driven oncogenesis independent of its reverse transcriptase activity. J. Clin. Investig. 2015, 125, 2109–2122.
- Park, J.I.; Venteicher, A.S.; Hong, J.Y.; Choi, J.; Jun, S.; Shkreli, M.; Chang, W.; Meng, Z.; Cheung, P.; Ji, H.; et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 2009, 460, 66–72.
- Pennington, K.L.; DeAngelis, M.M. Epidemiology of age-related macular degeneration (AMD): Associations with cardiovascular disease phenotypes and lipid factors. Eye Vis 2016, 3, 34.
- Bellezza, I. Oxidative Stress in Age-Related Macular Degeneration: Nrf2 as Therapeutic Target. Front. Pharm. 2018, 9, 1280.
- Cousins, S.W.; Espinosa-Heidmann, D.G.; Alexandridou, A.; Sall, J.; Dubovy, S.; Csaky, K. The role of aging, high fat diet and blue light exposure in an experimental mouse model for basal laminar deposit formation. Exp. Eye. Res. 2002, 75, 543–553.
- Heesterbeek, T.J.; Lorés-Motta, L.; Hoyng, C.B.; Lechanteur, Y.T.E.; den Hollander, A.I. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol Opt. 2020, 40, 140–170.
- Hughes, J.M.; Groot, A.J.; van der Groep, P.; Sersansie, R.; Vooijs, M.; van Diest, P.J.; Van Noorden, C.J.; Schlingemann, R.O.; Klaassen, I. Active HIF-1 in the normal human retina. J. Histochem. Cytochem. 2010, 58, 247–254.
- Sohal, R.S.; Mockett, R.J.; Orr, W.C. Mechanisms of aging: An appraisal of the oxidative stress hypothesis. Free. Radic. Biol. Med. 2002, 33, 575–586.
- Vatner, S.F.; Zhang, J.; Oydanich, M.; Berkman, T.; Naftalovich, R.; Vatner, D.E. Healthful Aging Mediated by Inhibition of Oxidative Stress. Ageing Res. Rev. 2020, 101194.
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye. Res. 2020, 100858.
- Drigeard Desgarnier, M.C.; Zinflou, C.; Mallet, J.D.; Gendron, S.P.; Méthot, S.J.; Rochette, P.J. Telomere Length Measurement in Different Ocular Structures: A Potential Implication in Corneal Endothelium Pathogenesis. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5547–5555.
- Nishio, M.; Tsukakoshi, K.; Ikebukuro, K. G-quadruplex: Flexible conformational changes by cations, pH, crowding and its applications to biosensing. Biosens. Bioelectron. 2021, 178, 113030.
- Shafirovich, V.; Geacintov, N.E. Excision of Oxidatively Generated Guanine Lesions by Competitive DNA Repair Pathways. Int. J. Mol. Sci. 2021, 22, 2698.
- Banevicius, M.; Gedvilaite, G.; Vilkeviciute, A.; Kriauciuniene, L.; Zemaitiene, R.; Liutkeviciene, R. Association of relative leukocyte telomere length and genetic variants in telomere-related genes (TERT, TERT-CLPTM1, TRF1, TNKS2, TRF2) with atrophic age-related macular degeneration. Ophthalmic Genet. 2021, 42, 189–194.
- Bodnar, A.G.; Ouellette, M.; Frolkis, M.; Holt, S.E.; Chiu, C.P.; Morin, G.B.; Harley, C.B.; Shay, J.W.; Lichtsteiner, S.; Wright, W.E. Extension of life-span by introduction of telomerase into normal human cells. Science 1998, 279, 349–352.
- Blasiak, J.; Glowacki, S.; Kauppinen, A.; Kaarniranta, K. Mitochondrial and nuclear DNA damage and repair in age-related macular degeneration. Int. J. Mol. Sci. 2013, 14, 2996–3010.
- Blasiak, J.; Pawlowska, E.; Szczepanska, J.; Kaarniranta, K. Interplay between Autophagy and the Ubiquitin-Proteasome System and Its Role in the Pathogenesis of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 210.
- Kaarniranta, K.; Sinha, D.; Blasiak, J.; Kauppinen, A.; Vereb, Z.; Salminen, A.; Boulton, M.E.; Petrovski, G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy 2013, 9, 973–984.
- Ahmed, S.; Passos, J.F.; Birket, M.J.; Beckmann, T.; Brings, S.; Peters, H.; Birch-Machin, M.A.; von Zglinicki, T.; Saretzki, G. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J. Cell Sci. 2008, 121, 1046–1053.
- Martens, A.; Schmid, B.; Akintola, O.; Saretzki, G. Telomerase Does Not Improve DNA Repair in Mitochondria upon Stress but Increases MnSOD Protein under Serum-Free Conditions. Int. J. Mol. Sci. 2019, 21, 27.
- Singhapol, C.; Pal, D.; Czapiewski, R.; Porika, M.; Nelson, G.; Saretzki, G.C. Mitochondrial telomerase protects cancer cells from nuclear DNA damage and apoptosis. PLoS ONE 2013, 8, e52989.
- Sharma, G.G.; Gupta, A.; Wang, H.; Scherthan, H.; Dhar, S.; Gandhi, V.; Iliakis, G.; Shay, J.W.; Young, C.S.; Pandita, T.K. hTERT associates with human telomeres and enhances genomic stability and DNA repair. Oncogene 2003, 22, 131–146.
- Liang, H.; Ward, W.F. PGC-1alpha: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151.
- Austin, S.; St-Pierre, J. PGC1α and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 2012, 125, 4963–4971.
- Supruniuk, E.; Mikłosz, A.; Chabowski, A. The Implication of PGC-1α on Fatty Acid Transport across Plasma and Mitochondrial Membranes in the Insulin Sensitive Tissues. Front. Physiol. 2017, 8.
- Diao, J.; Zhao, H.; You, P.; You, H.; Wu, H.; Shou, X.; Cheng, G. Rosmarinic acid ameliorated cardiac dysfunction and mitochondrial injury in diabetic cardiomyopathy mice via activation of the SIRT1/PGC-1α pathway. Biochem. Biophys. Res. Commun. 2021, 546, 29–34.
- Hao, Q.; Zheng, A.; Zhang, H.; Cao, H. Down-regulation of betatrophin enhances insulin sensitivity in type 2 diabetes mellitus through activation of the GSK-3β/PGC-1α signaling pathway. J. Endocrinol. Investig. 2021.
- Kim, J.; Moon, J.; Park, C.H.; Lee, J.; Cheng, H.; Floyd, Z.E.; Chang, J.S. NT-PGC-1α deficiency attenuates high-fat diet-induced obesity by modulating food intake, fecal fat excretion and intestinal fat absorption. Sci. Rep. 2021, 11, 1323.
- Geng, T.; Li, P.; Yin, X.; Yan, Z. PGC-1α promotes nitric oxide antioxidant defenses and inhibits FOXO signaling against cardiac cachexia in mice. Am. J. Pathol. 2011, 178, 1738–1748.
- Guo, A.; Li, K.; Xiao, Q. Fibroblast growth factor 19 alleviates palmitic acid-induced mitochondrial dysfunction and oxidative stress via the AMPK/PGC-1α pathway in skeletal muscle. Biochem. Biophys. Res. Commun. 2020, 526, 1069–1076.
- Tormos, A.M.; Pérez-Garrido, S.; Taléns-Visconti, R.; Nebreda, Á.R.; Sastre, J. Long term p38-a deficiency up-regulates antioxidant enzymes through compensatory NF-κB activation. Free. Radic. Biol Med. 2014, 75 (Suppl. S1), S52.
- Zhang, Y.; Wang, C.; Jin, Y.; Yang, Q.; Meng, Q.; Liu, Q.; Dai, Y.; Cai, L.; Liu, Z.; Liu, K.; et al. Activating the PGC-1α/TERT Pathway by Catalpol Ameliorates Atherosclerosis via Modulating ROS Production, DNA Damage, and Telomere Function: Implications on Mitochondria and Telomere Link. Oxidative Med. Cell. Longev. 2018, 2018, 2876350.
- Brown, E.E.; DeWeerd, A.J.; Ildefonso, C.J.; Lewin, A.S.; Ash, J.D. Mitochondrial oxidative stress in the retinal pigment epithelium (RPE) led to metabolic dysfunction in both the RPE and retinal photoreceptors. Redox Biol. 2019, 24, 101201.
- Kaarniranta, K.; Kajdanek, J.; Morawiec, J.; Pawlowska, E.; Blasiak, J. PGC-1α Protects RPE Cells of the Aging Retina against Oxidative Stress-Induced Degeneration through the Regulation of Senescence and Mitochondrial Quality Control. The Significance for AMD Pathogenesis. Int. J. Mol. Sci. 2018, 19, 2317.
- Ruan, Y.; Jiang, S.; Gericke, A. Age-Related Macular Degeneration: Role of Oxidative Stress and Blood Vessels. Int. J. Mol. Sci. 2021, 22, 1296.
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W., Jr.; Ding, J.; et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 2014, 10, 1989–2005.
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega Á, L.; Pérez, S. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxidative Med. Cell. Longev. 2020, 2020, 1452696.
