There is a lack of evidence that phenobarbital or any other anticonvulsant improves outcomes after neonatal seizures have already started. However, there is some clinical and preclinical evidence that prophylactic phenobarbital administration, that is to say administered before the appearance of seizure activity, may be beneficial
[25][68]. Any benefit could be mediated by improved seizure control as phenobarbital suppressed seizures when administered before, but not after hypoxia-ischemia in P11 rats (
Table 1)
[26][38]. Although brain injury was not assessed in this study
[26][38], there is intriguing evidence that phenobarbital may have neuroprotective effects independent of its anticonvulsant properties. For example, a small prospective study, conducted in the pre-hypothermia era, found that prophylactic high-dose phenobarbital administration (40 mg/kg) was associated with improved neurological outcome at three years of age, despite only showing a 27% reduction in seizures compared to neonates who received phenobarbital after seizures had started
[27][59]. Further supporting the efficacy of prophylactic phenobarbital, a recent Cochrane review of prophylactic barbiturates (8/9 studies used phenobarbital) for infants with perinatal HIE
[25][68], found that prophylactic barbiturate therapy reduced the risk of seizures after hypoxia-ischemia, with no reduction in mortality. However, there was little data on long-term outcomes. They concluded that
“the results of the current review support the use of prophylactic barbiturate therapy as a promising area of research” although they could not recommend routine clinical use at this stage due to the low quality of evidence.
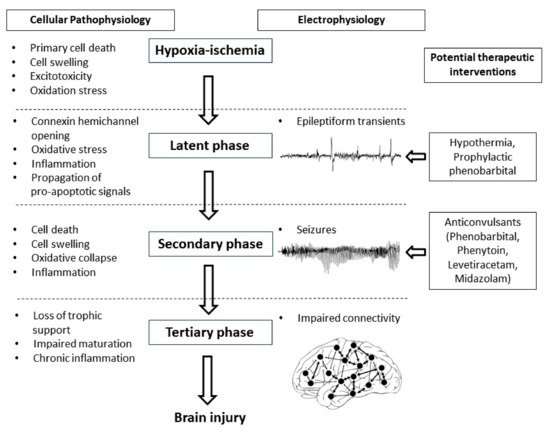
The potential for early but not later administration of phenobarbital to be neuroprotective, likely relates to the well-characterized progressive evolution of hypoxic-ischemic brain injury (
Figure 1)
[3]. During the period of hypoxia-ischemia itself, there is often only limited cell death. Many brain cells partially or even completely recover after reperfusion, in a latent phase that lasts approximately 6 h after hypoxia-ischemia, only to undergo secondary deterioration as shown by delayed onset of seizures, cell swelling and bulk cell death from 6–72 h after hypoxia-ischemia
[3]. It is now well established that therapeutic hypothermia needs to be started during this latent phase, before the onset of seizures (and secondary cell death), in order to be effective
[28][69]. This suggests that when phenobarbital is administered after the onset of seizures (i.e., during the secondary phase), the window of opportunity for neuroprotection has already closed and so phenobarbital can only act as an anticonvulsant. It is plausible but unproven that administration of prophylactic phenobarbital during the latent phase, corresponding with the established window of opportunity for therapeutic hypothermia
[28][69], may offer direct neuroprotection, and so reduce secondary cell death, leading to fewer seizures.
Figure 1. Flow diagram showing the phases of injury including hypoxia-ischemia, the latent phase, the secondary phase and the tertiary phase leading to the development of brain injury. Intervention during the latent phase with therapeutic hypothermia or prophylactic phenobarbital has the potential to reduce the development of brain injury as well as the occurrence of seizures. Intervention during the secondary phase with anticonvulsants such as phenobarbital, phenytoin, levetiracetam and midazolam may reduce seizure activity but their effects on long-term outcome are not clear.
There is some evidence to suggest that early phenobarbital can augment the beneficial effects of therapeutic hypothermia. In P7 rats exposed to hypoxia-ischemia induced by single carotid artery ligation and inhalation hypoxia, phenobarbital augmented hypothermic neuroprotection
[29][39]. Similarly, in P10 rat pups, Krishna et al. also found augmentation of hypothermic neuroprotection with prophylactic phenobarbital subjected to unilateral hypoxia-ischemia
[30][40]. Although these studies are limited by the use of sub-optimal durations of hypothermia (3 to 4 h instead of the 72 h in established clinical protocols), they support the concept that prophylactic phenobarbital administration may have additive neuroprotective effects with therapeutic hypothermia. Strikingly, there no large animal, translational studies to date have tested whether there is benefit from combined treatment with prophylactic phenobarbital and hypothermia.
5. Conclusions
Current anticonvulsant protocols are clearly far from optimal for use in neonates—they show limited efficacy, have high potential for adverse effects and there is a striking lack of evidence that they improve long-term outcomes. Preclinical neurophysiological studies are essential to help to better understand the pathophysiology of seizures occurring in the neonatal brain compared to the mature brain and identify specific anticonvulsant treatment strategies for the neonatal brain. High quality translational studies will help to answer questions around whether prophylactic anticonvulsant treatment is neuroprotective and more effective than delayed administration and whether anticonvulsants are associated with neurotoxic effects when given in combination with therapeutic hypothermia. Well-designed randomized controlled trials, taking into account the use of therapeutic hypothermia, are essential to develop evidence-based treatment strategies for the neonate with seizures.