Lung cancer is one of the most common and malignant cancers with extremely high morbidity and mortality in both males and females. Although traditional lung cancer treatments are fast progressing, there are still limitations. Caveolin-1 (Cav-1), a main component of caveolae, participates in multiple cellular events such as immune responses, endocytosis, membrane trafficking, cellular signaling and cancer progression. It has been found tightly associated with lung cancer cell proliferation, migration, apoptosis resistance and drug resistance. In addition to this, multiple bioactive molecules have been confirmed to target Cav-1 to carry on their anti-tumor functions in lung cancers. Cav-1 can also be a predictor for lung cancer patients’ prognosis.
- Caveolin-1 (Cav-1)
- lung cancer
- tumor progression
- metastasis
- targeted therapy
1. Introduction
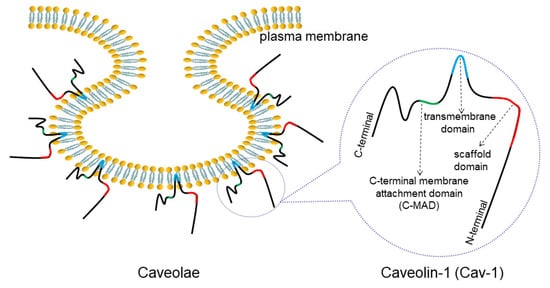
2. Overview of Caveolae and Cav-1
| Tissue Type | Histological Type | Tumor Grade | Cav-1 positive Number/Total Sample Number | Reference |
|---|---|---|---|---|
| Non-cancer tissue | ----- | ----- | 16/16(100%) | [29] |
| 15/19(78.9%) | [30] | |||
| 20/20(100%) | [31] | |||
| 20/20(100%) | [32] | |||
| Lung cancer tissue | AC | IV | 23/116(19.8%) | [25] |
5.1. Cav-1 and Drug Resistance
5.2. Cav-1 and Cell Senescence
5.3. Multiple Cav-1-Targeted Agents
| Agent | Cell Line | Mode of Action | Reference |
|---|---|---|---|
| H2O2 and O2·− | H460 | H2O2 and O2·−→ Cav-1↓→ pAKT↓→ migration↓ | [90] |
| Chrysotobibenzyl | H460 H292 | Chrysotobibenzyl→ Cav-1↓→ integrin β1, β3, αv↓ → pFAK↓ and pAKT↓→ migration↓ |
[72] |
| Gigantol | H460 | Gigantol→ Cav-1↓→ pAKT↓→ CDC42↓→ EMT↓ and migration↓ | [115] |
| DF-A | H460 | DF-A→ Cav-1↓, Mcl-1↓and Bcl-2↓→ anoikis↑ | [122] |
| Moscatilin | H460 | Moscatilin→ Cav-1↓→ Mcl-1↓→ pAKT↓,pERK↓→ anoikis↑ |
[117] |
| AC | I–III | 19/43(44.19%) | [29] |
| SCC | I–III | 34/107(31.7%) | [33] |
| AC+SCC+others (LCLC, ASC and carcinoid) | I–IV | 60/115(52.2%) | [30] |
| AC+SCC+LCLC | I–IV | 105/160(65.7%) | [31] |
| AC+SCC+LCLC | III and/or IV | 12/73(16.4%) | [34] |
| AC+SCC+LCLC+ASC | I–III | 69/140(49.3%) | [32] |
| SCLC | I–IV | 49/70(70%) | [35] |
3. Cav-1 Regulates Lung Cancer Cell Proliferation
4. Cav-1 Participates in Lung Cancer Metastasis
Cell migration is a process that can take place in both physiological and pathological conditions, such as early embryo development, tissue repair, immune response, inflammatory response and cancer metastasis [45]. Metastasis consists of a series of events that can be described as follows: cancer cells detach from extracellular matrix (ECM), then invade locally into the stroma by secreting matrix metalloproteinases (MMPs) to degrade ECM, later intravasate into blood or lymphatic vessels and survive within the circulation, and finally the cells extravasate and generate a metastatic lesion at a distant site (Figure 2) [46][47]. There are three different routes for metastasis, namely, direct seeding, the hematogenous pathway and lymphocytic pathway. Lung cancer undergoes metastasis through either blood vessels or lymphatic vessels in most cases. The most common sites that the secondary lung cancer preferentially occurs are bones, liver, brain and adrenal glands [48]. A clinicopathological test has unveiled that Cav-1-positive lung AC patients tended to have worse tumor, node, and metastasis (TNM) stage in comparison to the negative patients [30]. Hence, Cav-1 may play a significant role in lung cancer cell migration.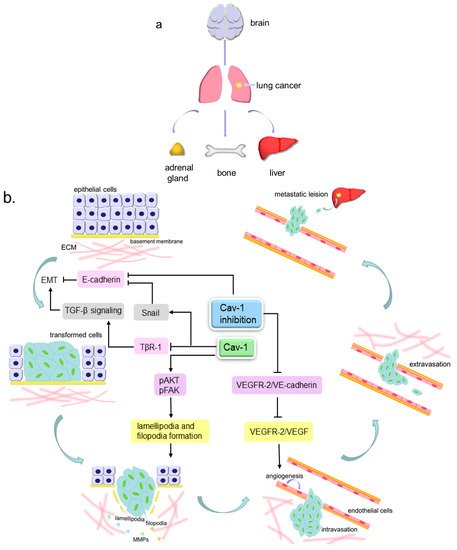
4.1. Cav-1 and Angiogenesis
4.2. Cav-1 and Pseudopods Formation
4.3. Cav-1 and EMT
4.4. Cav-1 Regulates Cell Movement via Multiple Molecules
5. Cav-1 Involved in Lung Cancer Therapy
Current treatments of lung cancer include surgery, chemotherapy, radiotherapy and targeted therapy. The traditional surgical resection of the tumor is generally recommended for early-stage lung cancer (especially NSCLC) patients [| Zinc | |||
| H460 | |||
| Zinc→ Cav-1↓→ pAKT↓→ anoikis↑ | |||
| [ | 118 | ] | |
| Cordycepin | A549 | Cordycepin→ Cav-1↑→ p-JNK↑→ Foxo3a↑→ Bax↑→ apoptosis↑ |
[119] |
| Jorunnamycin A | H460 H292 H23 |
Jorunnamycin A→ Cav-1↓→ pAKT↓,pERK↓→ EMT↓ and apoptosis↑ |
[120] |
| EEAC | A549 | EEAC→ Cav-1↓→ chemosensitivity to paclitaxel↑ | [121] |
| Albumin-encapsuled fenretinide | A549 | Cav-1 promotes albumin-encapsuled fenretinide uptake into cell→ apoptosis↑ | [103] |
| Bleomycin | A549 | Bleomycin→ Cav-1↑→ p53↑, p21↑→ senescence↑ | [112] |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Dutkowska, A.E.; Antczak, A. Comorbidities in lung cancer. Adv. Respir. Med. 2016, 84, 186–192.
- Knight, S.B.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and prospects of early detection in lung cancer. Open Biol. 2017, 7.
- Smart, E.J.; Graf, G.A.; McNiven, M.A.; Sessa, W.C.; Engelman, J.A.; Scherer, P.E.; Okamoto, T.; Lisanti, M.P. Caveolins, Liquid-Ordered Domains, and Signal Transduction. Mol. Cell. Biol. 1999, 19, 7289–7304.
- Echarri, A.; del Pozo, M.A. Caveolae–mechanosensitive membrane invaginations linked to actin filaments. J. Cell Sci. 2015, 128, 2747–2758.
- Parton, R.G.; Pozo, M.A. Caveolae as plasma membrane sensors, protectors and organizers. Nat. Rev. Mol. Cell Biol. 2013, 14, 98–112.
- Sohn, J.; Brick, R.M.; Tuan, R.S. From embryonic development to human diseases: The functional role of caveolae/caveolin. Birth Defects Res. Part C Embryo Today Rev. 2016, 108, 45–64.
- Yin, H.; Liu, T.; Zhang, Y.; Yang, B. Caveolin proteins: A molecular insight into disease. Front. Med. 2016, 10, 397–404.
- Nguyen, K.C.T.; Cho, K.A. Versatile Functions of Caveolin-1 in Aging-related Diseases. Chonnam Med. J. 2017, 53, 28–36.
- Williams, T.M.; Lisanti, M.P. The caveolin proteins. Genome Biol. 2004, 5, 1–8.
- Hansen, C.G.; Nichols, B.J. Exploring the caves: Cavins, caveolins and caveolae. Trends Cell Biol. 2010, 20, 177–186.
- Kovtun, O.; Tillu, V.A.; Ariotti, N.; Parton, R.G.; Collins, B.M. Cavin family proteins and the assembly of caveolae. J. Cell Sci. 2015, 128, 1269–1278.
- Yamaguchi, T.; Lu, C.; Ida, L.; Yanagisawa, K.; Usukura, J.; Cheng, J.; Hotta, N.; Shimada, Y.; Isomura, H.; Suzuki, M.; et al. ROR1 sustains caveolae and survival signalling as a scaffold of cavin-1 and caveolin-1. Nat. Commun. 2016, 7, 1–13.
- Busija, A.R.; Patel, H.H.; Insel, P.A. Caveolins and cavins in the trafficking, maturation, and degradation of caveolae: Implications for cell physiology. Am. J. Physiol. Cell Physiol. 2017, 312, C459–C477.
- Engelman, J.A.; Zhang, X.L.; Lisanti, M.P. Genes encoding human caveolin-1 and -2 are co-localized to the D7S522 locus (7q31. 1), a known fragile site (FRA7G) that is frequently deleted in human cancers. FEBS Lett. 1998, 436, 403–410.
- Goetz, J.G.; Lajoie, P.; Wiseman, S.M.; Nabi, I.R. Caveolin-1 in tumor progression: The good, the bad and the ugly. Cancer Metastasis Rev. 2008, 27, 715–735.
- Torrejón, B.; Cristóbal, I.; Rojo, F.; García-Foncillas, J. Caveolin-1 is Markedly Downregulated in Patients with Early-Stage Colorectal Cancer. World J. Surg. 2017, 41, 2625–2630.
- Wiechen, K.; Diatchenko, L.; Agoulnik, A.; Scharff, K.M.; Schober, H.; Arlt, K.; Zhumabayeva, B.; Siebert, P.D.; Dietel, M.; Schäfer, R.; et al. Caveolin-1 is down-regulated in human ovarian carcinoma and acts as a candidate tumor suppressor gene. Am. J. Pathol. 2001, 159, 1635–1643.
- Diaz-valdivia, N.; Bravo, D.; Huerta, H.; Henriquez, S.; Gabler, F.; Vega, M.; Romero, C.; Calderon, C.; Owen, G.I.; Leyton, L.; et al. Enhanced caveolin-1 expression increases migration, anchorage-independent growth and invasion of endometrial adenocarcinoma cells. BMC Cancer 2015, 15, 1–11.
- Tang, Y.; Zeng, X.; He, F.; Liao, Y.; Qian, N.; Toi, M. Caveolin-1 is related to invasion, survival, and poor prognosis in hepatocellular cancer. Med. Oncol. 2012, 29, 977–984.
- Yang, G.; Truong, L.D.; Timme, T.L.; Ren, C.; Wheeler, T.M.; Park, S.H.; Nasu, Y.; Bangma, C.H.; Kattan, M.W.; Scardino, P.T.; et al. Elevated expression of caveolin is associated with prostate and breast cancer. Clin. Cancer Res. 1998, 4, 1873–1880.
- Demirci, N.S.; Dogan, M.; Erdem, G.U.; Kacar, S.; Turhan, T.; Kilickap, S.; Cigirgan, L.C.; Kayacetin, E.; Bozkaya, Y.; Zengin, N. Is plasma caveolin-1 level a prognostic biomarker in metastatic pancreatic cancer? Saudi J. Gastroenterol. 2017, 23, 183–189.
- Chen, D.; Shen, C.; Du, H.; Zhou, Y.; Che, G. Duplex value of caveolin-1 in non-small cell lung cancer: A meta analysis. Fam. Cancer 2014, 13, 449–457.
- Kato, T.; Miyamoto, M.; Kato, K.; Cho, Y.; Itoh, T.; Morikawa, T.; Okushiba, S.; Kondo, S.; Ohbuchi, T.; Katoh, H. Difference of caveolin-1 expression pattern in human lung neoplastic tissue. Atypical adenomatous hyperplasia, adenocarcinoma and squamous cell carcinoma. Cancer Lett. 2004, 214, 121–128.
- Duregon, E.; Senetta, R.; Bertero, L.; Bussolati, B.; Annaratone, L.; Pittaro, A.; Papotti, M.; Marchiò, C.; Cassoni, P. Caveolin 1 expression favors tumor growth and is associated with poor survival in primary lung adenocarcinomas. Tumor Biol. 2017, 39, 1–7.
- Luan, T.Y.; Zhu, T.N.; Cui, Y.J.; Zhang, G.; Song, X.J.; Gao, D.M.; Zhang, Y.M.; Zhao, Q.L.; Liu, S.; Su, T.Y.; et al. Expression of caveolin-1 is correlated with lung adenocarcinoma proliferation, migration, and invasion. Med. Oncol. 2015, 32, 1–11.
- Sun, M.Z.; Guan, Z.; Liu, S.; Zhou, X.; Wangm, N.; Shao, S.; Lin, D. Caveolin-1 interferes cell growth of lung cancer NCI-H446 cell through the interactions with phospho-ERK1/2, estrogen receptor and progestin receptor. Biomed. Pharmacother. 2012, 66, 242–248.
- Sanuphan, A.; Chunhacha, P.; Pongrakhananon, V.; Chanvorachote, P. Long-Term Nitric Oxide Exposure Enhances Lung Cancer Cell Migration. Biomed. Res. Int. 2013, 2013.
- Ma, H.; Chen, H.; Zhu, R.; Zheng, M.; Liu, Y.; Fan, K.; Yang, W. Expression of caveolin-1 and its significance in the prognosis of lung squamous cell carcinoma. Chin. J. Clin. Oncol. 2013, 40, 93–96.
- Zhan, P.; Shen, X.; Qian, Q.; Wang, Q.; Zhu, J.; Zhang, Y.; Xie, H.; Xu, C.; Hao, K.; Hu, W.; et al. Expression of caveolin-1 is correlated with disease stage and survival in lung adenocarcinomas. Oncol. Rep. 2012, 27, 1072–1078.
- Liu, H.; Xing, L.; Wang, H.; Yang, J.; Sun, Y. Relationship between expression of caveolin-1 and pERK1/2 and prognosis in non-small cell lung cancer. Chin. J. Pathol. 2008, 37, 615–619.
- Chen, H.L.; Fan, L.F.; Gao, J.; Ouyang, J.P.; Zhang, Y.X. Differential expression and function of the caveolin-1 gene in non-small cell lung carcinoma. Oncol. Rep. 2011, 25, 359–366.
- Yoo, S.H.; Park, Y.S.; Kim, H.R.; Sung, S.W.; Kim, J.H.; Shim, Y.S.; Lee, S.D.; Choi, Y.L.; Kim, M.K.; Chung, D.H. Expression of caveolin-1 is associated with poor prognosis of patients with squamous cell carcinoma of the lung. Lung Cancer 2003, 42, 195–202.
- Ho, C.C.; Kuo, S.H.; Huang, P.H.; Huang, H.Y.; Yang, C.H.; Yang, P.C. Caveolin-1 expression is significantly associated with drug resistance and poor prognosis in advanced non-small cell lung cancer patients treated with gemcitabine-based chemotherapy. Lung Cancer 2008, 59, 105–110.
- Wu, J.; Di, D.; Zhao, C.; Pan, Q.; Liu, Y.; Zhang, X.; Zhao, X.; Chen, H. Clinical significance of Gli-1 and Caveolin-1 expression in the human small cell lung cancer. Asian Pac. J. Cancer Prev. 2018, 19, 401–406.
- Vijayaraghavan, S.; Moulder, S.; Keyomarsi, K.; Layman, R.M. Inhibiting CDK in Cancer Therapy: Current Evidence and Future Directions. Target. Oncol. 2018, 13, 21–38.
- Diaz-Moralli, S.; Tarrado-Castellarnau, M.; Miranda, A.; Cascante, M. Targeting cell cycle regulation in cancer therapy. Pharmacol. Ther. 2013, 138, 255–271.
- Golias, C.H.; Charalabopoulos, A.; Charalabopoulos, K. Cell proliferation and cell cycle control: A mini review. Int. J. Clin. Pract. 2004, 58, 1134–1141.
- Song, Y.; Xue, L.; Du, S.; Sun, M.; Hu, J.; Hao, L.; Gong, L.; Yeh, D.; Xiong, H.; Shao, S. Caveolin-1 knockdown is associated with the metastasis and proliferation of human lung cancer cell line NCI-H460. Biomed. Pharmacother. 2012, 66, 439–447.
- Han, F.; Zhang, L.; Zhou, Y.; Yi, X. Caveolin-1 regulates cell apoptosis and invasion ability in paclitaxel-induced multidrug-resistant A549 lung cancer cells. Int. J. Clin. Exp. Pathol. 2015, 8, 8937–8947.
- Pancotti, F.; Roncuzzi, L.; Maggiolini, M.; Gasperi-Campani, A. Caveolin-1 silencing arrests the proliferation of metastatic lung cancer cells through the inhibition of STAT3 signaling. Cell Signal 2012, 24, 1390–1397.
- Tian, J.M.; Ran, B.; Zhang, C.L.; Yan, D.M.; Li, X.H. Estrogen and progesterone promote breast cancer cell proliferation by inducing cyclin G1 expression. Braz. J. Med. Biol. Res. Rev. Braz. Pesqui Med. Biol. 2018, 51, 1–7.
- Bazzani, L.; Donnini, S.; Giachetti, A.; Christofori, G.; Ziche, M. PGE2 mediates EGFR internalization and nuclear translocation via caveolin endocytosis promoting its transcriptional activity and proliferation in human NSCLC cells. Oncotarget 2018, 9, 14939–14958.
- Liu, W.; Yin, N.; Liu, H.; Nan, K. Cav-1 promote lung cancer cell proliferation and invasion through lncRNA HOTAIR. Gene 2018, 641, 335–340.
- Jacquemet, G.; Hamidi, H.; Ivaska, J. Filopodia in cell adhesion, 3D migration and cancer cell invasion. Curr. Opin. Cell Biol. 2015, 36, 23–31.
- Wood, S.L.; Pernemalm, M.; Crosbie, P.A.; Whetton, A.D. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat. Rev. 2014, 40, 558–566.
- Perlikos, F.; Harrington, K.J.; Syrigos, K.N. Key molecular mechanisms in lung cancer invasion and metastasis: A comprehensive review. Crit. Rev. Oncol. Hematol. 2013, 87, 1–11.
- Popper, H.H. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016, 35, 75–91.
- Bielenberg, D.R.; Zetter, B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. (U. S.) 2015, 21, 267–273.
- Bremnes, R.M.; Dønnem, T.; Al-Saad, S.; Al-Shibli, K.; Andersen, S.; Sirera, R.; Camps, C.; Marinez, I.; Busund, L.T. The role of tumor stroma in cancer progression and prognosis: Emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 209–217.
- Erdogan, B.; Webb, D.J. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem. Soc. Trans. 2017, 45, 229–236.
- Gratton, J.P.; Lin, M.I.; Yu, J.; Weiss, E.D.; Jiang, Z.L.; Fairchild, T.A.; Iwakiri, Y.; Groszmann, R.; Claffey, K.P.; Cheng, Y.C.; et al. Selective inhibition of tumor microvascular permeability by cavtratin blocks tumor progression in mice. Cancer Cell 2003, 4, 31–39.
- Claesson-Welsh, L.; Welsh, M. VEGFA and tumour angiogenesis. J. Intern. Med. 2013, 273, 114–127.
- Shahneh, F.Z.; Baradaran, B.; Zamani, F.; Aghebati-Maleki, L. Tumor angiogenesis and anti-angiogenic therapies. Hum. Antibodies 2013, 22, 15–19.
- Dimova, I.; Popivanov, G.; Djonov, V. Angiogenesis in cancer - General pathways and their therapeutic implications. J. BUON 2014, 19, 15–21.
- García-Cardeña, G.; Martasek, P.; Masters, B.S.S.; Skidd, P.M.; Couet, J.; Li, S.; Lisanti, M.P.; Sessa, W.C. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J. Biol. Chem. 1997, 272, 25437–25440.
- Schmitz, M.; Zerr, I.; Althaus, H.H. Effect of cavtratin, a caveolin-1 scaffolding domain peptide, on oligodendroglial signaling cascades. Cell Mol. Neurobiol. 2011, 31, 991–997.
- Dong, J.; Cheng, M.; Sun, H. Function of inducible nitric oxide synthase in the regulation of cervical cancer cell proliferation and the expression of vascular endothelial growth factor. Mol. Med. Rep. 2014, 9, 583–589.
- Lin, M.I.; Yu, J.; Murata, T.; Sessa, W.C. Caveolin-1 - Deficient mice have increased tumor microvascular permeability, angiogenesis, and growth. Cancer Res. 2007, 67, 2849–2856.
- Falcon, B.L.; Chintharlapalli, S.; Uhlik, M.T.; Pytowski, B. Antagonist antibodies to vascular endothelial growth factor receptor 2 (VEGFR-2) as anti-angiogenic agents. Pharmacol. Ther. 2016, 164, 204–225.
- Om Alblazi, K.M.; Siar, C.H. Cellular protrusions - Lamellipodia, filopodia, invadopodia and podosomes - and their roles in progression of orofacial tumours: Current understanding. Asian Pac. J. Cancer Prev. 2015, 16, 2187–2191.
- Lawson, C.; Lim, S.T.; Uryu, S.; Chen, X.L.; Calderwood, D.A.; Schlaepfer, D.D. FAK promotes recruitment of talin to nascent adhesions to control cell motility. J. Cell Biol. 2012, 196, 223–232.
- Hu, Y.L.; Lu, S.; Szeto, K.W.; Sun, J.; Wang, Y.; Lasheras, J.C.; Chien, S. FAK and paxillin dynamics at focal adhesions in the protrusions of migrating cells. Sci. Rep. 2014, 4, 1–7.
- Yu, H.; Gao, M.; Ma, Y.; Wang, L.; Shen, Y.; Liu, X. Inhibition of cell migration by focal adhesion kinase: Time-dependent difference in integrin-induced signaling between endothelial and hepatoblastoma cells. Int. J. Mol. Med. 2018, 41, 2573–2588.
- Tureèková, J.; Vojtìchová, M.; Krausová, M.; Šloncová, E.; Koøínek, V. Focal adhesion kinase functions as an Akt downstream target in migration of colorectal cancer cells. Transl. Oncol. 2009, 2, 281–290.
- Enomoto, A.; Murakami, H.; Asai, N.; Morone, N.; Watanabe, T.; Kawai, K.; Murakumo, Y.; Usukura, J.; Kaibuchi, K.; Takahashi, M. Akt/PKB regulates actin organization and cell motility via girdin/APE. Dev. Cell 2005, 9, 389–402.
- Enomoto, A.; Ping, J.; Takahashi, M. Girdin, a novel actin-binding protein, and its family of proteins possess versatile functions in the Akt and Wnt signaling pathways. Ann. N. Y. Acad. Sci. 2006, 1086, 169–184.
- Rottner, K.; Faix, J.; Bogdan, S.; Linder, S.; Kerkhoff, E. Actin assembly mechanisms at a glance. J. Cell Sci. 2017, 130, 3427–3435.
- Yamaguchi, H.; Condeelis, J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 642–652.
- Chanvorachote, P.; Chunhacha, P.; Pongrakhananon, V. Caveolin-1 induces lamellipodia formation via an Akt-dependent pathway. Cancer Cell Int. 2014, 14, 1–9.
- Ho, C.C.; Huang, P.H.; Huang, H.Y.; Chen, Y.H.; Yang, P.C.; Hsu, S.M. Up-regulated caveolin-1 accentuates the metastasis capability of lung adenocarcinoma by inducing filopodia formation. Am. J. Pathol. 2002, 161, 1647–1656.
- Petpiroon, N.; Bhummaphan, N.; Tungsukruthai, S.; Pinkhien, T.; Maiuthed, A.; Sritularak, B.; Chanvorachote, P. Chrysotobibenzyl inhibition of lung cancer cell migration through Caveolin-1-dependent mediation of the integrin switch and the sensitization of lung cancer cells to cisplatin-mediated apoptosis. Phytomedicine 2019, 58.
- Sung, W.J.; Kim, H.; Park, K.K. The biological role of epithelial-mesenchymal transition in lung cancer (Review). Oncol. Rep. 2016, 36, 1199–1206.
- Jolly, M.K.; Ward, C.; Eapen, M.S.; Myers, S.; Hallgren, O.; Levine, H.; Sohal, S.S. Epithelial–mesenchymal transition, a spectrum of states: Role in lung development, homeostasis, and disease. Dev. Dyn. 2018, 247, 346–358.
- Legras, A.; Pécuchet, N.; Imbeaud, S.; Pallier, K.; Didelot, A.; Roussel, H.; Gibault, L.; Fabre, E.; Le Pimpec-Barthes, F.; Laurent-Puig, P.; et al. Epithelial-to-mesenchymal transition and microRNAs in lung cancer. Cancers (Basel) 2017, 9, 101.
- Horejs, C.M. Basement membrane fragments in the context of the epithelial-to-mesenchymal transition. Eur. J. Cell Biol. 2016, 95, 427–440.
- Tae, N.; Lee, S.; Kim, O.; Park, J.; Na, S.; Lee, J.H. Syntenin promotes VEGF-induced VEGFR2 endocytosis and angiogenesis by increasing ephrin-B2 function in endothelial cells. Oncotarget 2017, 8, 38886–38901.
- Hwangbo, C.; Tae, N.; Lee, S.; Kim, O.; Park, O.K.; Kim, J.; Kwon, S.H.; Lee, J.H. Syntenin regulates TGF-â1-induced Smad activation and the epithelial-to-mesenchymal transition by inhibiting caveolin-mediated TGF-â type i receptor internalization. Oncogene 2016, 35, 389–401.
- Fan, C.C.; Cheng, W.C.; Huang, Y.C.; Sher, Y.P.; Liou, N.J.; Chien, Y.C.; Lin, P.S.; Lin, P.S.; Chen, C.H.; Chang, W.C. EFHD2 promotes epithelial-to-mesenchymal transition and correlates with postsurgical recurrence of stage I lung adenocarcinoma. Sci. Rep. 2017, 7, 1–11.
- Basu, S.; Cheriyamundath, S.; Ben-Ze’ev, A. Cell-cell adhesion: Linking Wnt/â-catenin signaling with partial EMT and stemness traits in tumorigenesis. F1000Research 2018, 7, 1–9.
- Barrallo-Gimeno, A.; Nieto, M.A. The Snail genes as inducers of cell movement and survival: Implications in development and cancer. Development 2005, 132, 3151–3161.
- Kim, Y.J.; Kim, J.H.; Kim, O.; Ahn, E.J.; Oh, S.J.; Akanda, M.R.; Oh, I.J.; Jung, S.; Kim, K.K.; Lee, J.H.; et al. Caveolin-1 enhances brain metastasis of non-small cell lung cancer, potentially in association with the epithelial-mesenchymal transition marker SNAIL. Cancer Cell Int. 2019, 19, 1–13.
- Han, F.; Zhang, J.; Shao, J.; Yi, X. Caveolin-1 promotes an invasive phenotype and predicts poor prognosis in large cell lung carcinoma. Pathol. Res. Pract. 2014, 210, 514–520.
- Liang, L.; Li, L.; Zeng, J.; Gao, Y.; Chen, Y.L.; Wang, Z.Q.; Wang, X.Y.; Chang, L.S.; He, D. Inhibitory effect of silibinin on EGFR signal-induced renal cell carcinoma progression via suppression of the EGFR/MMP-9 signaling pathway. Oncol. Rep. 2012, 28, 999–1005.
- Yeh, D.; Chen, C.; Sun, M.Z.; Shao, S.; Hao, L.; Song, Y.; Gong, L.; Hu, J.; Wang, Q. Caveolin-1 is an important factor for the metastasis and proliferation of human small cell lung cancer NCI-H446 cell. Anat. Rec. 2009, 292, 1584–1592.
- Sinha, B.; Köster, D.; Ruez, R.; Gonnord, P.; Bastiani, M.; Abankwa, D.; Stan, R.V.; Butler-Browne, G.; Vedie, B.; Johannes, L.; et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 2011, 144, 402–413.
- Kao, Y.C.; Jheng, J.R.; Pan, H.J.; Liao, W.Y.; Lee, C.H.; Kuo, P.L. Elevated hydrostatic pressure enhances the motility and enlarges the size of the lung cancer cells through aquaporin upregulation mediated by caveolin-1 and ERK1/2 signaling. Oncogene 2017, 36, 863–874.
- Zakrzewicz, D.; Didiasova, M.; Krüger, M.; Giaimo, B.D.; Borggrefe, T.; Mieth, M.; Hocke, A.C.; Zakrzewicz, A.; Schaefer, L.; Preissner, K.T.; et al. Protein arginine methyltransferase 5 mediates enolase-1 cell surface trafficking in human lung adenocarcinoma cells. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1816–1827.
- Chanvorachote, P.; Chunhacha, P. Caveolin-1 Regulates Endothelial Adhesion of Lung Cancer Cells via Reactive Oxygen Species-Dependent Mechanism. PLoS ONE 2013, 8, 1–10.
- Luanpitpong, S.; Talbott, S.J.; Rojanasakul, Y.; Nimmannit, U.; Pongrakhananon, V.; Wang, L.; Chanvorachote, P. Regulation of lung cancer cell migration and invasion by reactive oxygen species and caveolin-1. J. Biol. Chem. 2010, 285, 38832–38840.
- Du, X.; Qian, X.; Papageorge, A.; Schetter, A.J.; Vass, W.C.; Liu, X.; Braverman, R.; Robles, A.I.; Lowy, D.R. Functional interaction of tumor suppressor DLC1 and caveolin-1 in cancer cells. Cancer Res. 2012, 72, 4405–4416.
- Vansteenkiste, J.; Crinò, L.; Dooms, C.; Douillard, J.Y.; Faivre-Finn, C.; Lim, E.; Rocco, G.; Senan, S.; van Schil, P.; Veronesi, G.; et al. 2nd ESMO consensus conference on lung cancer: Early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, 1462–1474.
- Lemjabbar-Alaoui, H.; Hassan, O.U.I.; Yang, Y.W.; Buchanan, P. Lung cancer: Biology and treatment options. Biochim. Biophys. Acta Rev. Cancer 2015, 1856, 189–210.
- Du, L.; Morgensztern, D. Chemotherapy for Advanced-Stage Non–Small Cell Lung Cancer. Cancer J. 2015, 21, 366–370.
- Shtivelman, E.; Hensing, T.; Simon, G.R.; Dennis, P.A.; Otterson, G.A.; Bueno, R.; Salgia, R. Molecular pathways and therapeutic targets in lung cancer. Oncotarget 2014, 5, 1392–1433.
- Zhang, S.; Cao, W.; Yue, M.; Zheng, N.; Hu, T.; Yang, S.; Dong, Z.; Lu, S.; Mo, S. Caveolin-1 affects tumor drug resistance in esophageal squamous cell carcinoma by regulating expressions of P-gp and MRP1. Tumor Biol. 2016, 37, 9189–9196.
- Li, Z.; Wang, N.; Huang, C.; Bao, Y.; Jiang, Y.; Zhu, G. Downregulation of Caveolin-1 increases the sensitivity of drug-resistant colorectal cancer HCT116 cells to 5-Fluorouracil. Oncol. Lett. 2017, 13, 483–487.
- Ruan, H.L.; Li, X.; Yang, H.M.; Song, Z.S.; Tong, J.W.; Cao, Q.; Wang, K.S.; Xiao, W.; Xiao, H.B.; Chen, X.Y.; et al. Enhanced expression of caveolin-1 possesses diagnostic and prognostic value and promotes cell migration, invasion and sunitinib resistance in the clear cell renal cell carcinoma. Exp. Cell Res. 2017, 358, 269–278.
- Yang, C.P.H.; Galbiati, F.; Volonté, D.; Horwitz, S.B.; Lisanti, M.P. Upregulation of caveolin-1 and caveolae organelles in Taxol-resistant A549 cells. FEBS Lett. 1998, 439, 368–372.
- Cui, Y.; Zhu, T.; Song, X.; Liu, J.; Liu, S.; Zhao, R. Downregulation of caveolin-1 increased EGFR-TKIs sensitivity in lung adenocarcinoma cell line with EGFR mutation. Biochem. Biophys. Res. Commun. 2018, 495, 733–739.
- Wongvaranon, P.; Pongrakhananon, V.; Chunhacha, P.; Chanvorachote, P. Acquired resistance to chemotherapy in lung cancer cells mediated by prolonged nitric oxide exposure. Anticancer Res. 2013, 33, 5433–5444.
- Chatterjee, M.; Ben-Josef, E.; Robb, R.; Vedaie, M.; Seum, S.; Thirumoorthy, K.; Palanichamy, K.; Harbrecht, M.; Chakravarti, A.; Williams, T.M. Caveolae-mediated endocytosis is critical for albumin cellular uptake and response to albumin-bound chemotherapy. Cancer Res. 2017, 77, 5925–5937.
- Pignatta, S.; Orienti, I.; Falconi, M.; Teti, G.; Arienti, C.; Medri, L.; Zanoni, M.; Carloni, S.; Zoli, W.; Amadori, D.; et al. Albumin nanocapsules containing fenretinide: Pre-clinical evaluation of cytotoxic activity in experimental models of human non-small cell lung cancer. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 263–273.
- Binkhathlan, Z.; Lavasanifar, A. P-glycoprotein Inhibition as a Therapeutic Approach for Overcoming Multidrug Resistance in Cancer: Current Status and Future Perspectives. Curr. Cancer Drug Targets 2013, 13, 326–346.
- Mealey, K.L.; Fidel, J. P-Glycoprotein Mediated Drug Interactions in Animals and Humans with Cancer. J. Vet. Intern. Med. 2015, 29, 1–6.
- Demeule, M.; Jodoin, J.; Gingras, D.; Béliveau, R. P-glycoprotein is localized in caveolae in resistant cells and in brain capillaries. FEBS Lett. 2000, 466, 219–224.
- Cai, C.; Chen, J. Overexpression of caveolin-1 induces alteration of multidrug resistance in Hs578T breast adenocarcinoma cells. Int. J. Cancer 2004, 111, 522–529.
- Lee, C.Y.; Lai, T.Y.; Tsai, M.K.; Ou-Yang, P.; Tsai, C.Y.; Wu, S.W.; Hsu, L.C.; Chen, J.S. The influence of a caveolin-1 mutant on the function of P-glycoprotein. Sci. Rep. 2016, 6, 1–9.
- Campisi, J. Aging, Cellular Senescence, and Cancer. Annu. Rev. Physiol. 2013, 75, 685–705.
- Lee, S.; Lee, J.S. Cellular senescence: A promising strategy for cancer therapy. BMB Rep. 2019, 52, 35–41.
- Kasper, M.; Barth, K. Bleomycin and its Role in Inducing Apoptosis and Senescence in Lung Cells - Modulating Effects of Caveolin-1. Curr. Cancer Drug Targets 2009, 9, 341–353.
- Linge, A.; Weinhold, K.; Bläsche, R.; Kasper, M.; Barth, K. Downregulation of caveolin-1 affects bleomycin-induced growth arrest and cellular senescence in A549 cells. Int. J. Biochem. Cell Biol. 2007, 39, 1964–1974.
- Galbiati, F.; Volonte’, D.; Liu, J.; Capozza, F.; Frank, P.G.; Zhu, L.; Pestell, R.G.; Lisanti, M.P. Caveolin-1 expression negatively regulates cell cycle progression by inducing G0/G1 arrest via a p53/p21WAF1/Cip1-dependent mechanism. Mol. Biol. Cell 2001, 12, 2229–2244.
- Linge, A.; Meleady, P.; Henry, M.; Clynes, M.; Kasper, M.; Barth, K. Bleomycin treatment of A549 human lung cancer cells results in association of MGr1-Ag and caveolin-1 in lipid rafts. Int. J. Biochem. Cell Biol. 2011, 43, 98–105.
- Charoenrungruang, S.; Chanvorachote, P.; Sritularak, B.; Pongrakhananon, V. Gigantol, a bibenzyl from Dendrobium draconis, inhibits the migratory behavior of non-small cell lung cancer cells. J. Nat. Prod. 2014, 77, 1359–1366.
- Chunhacha, P.; Pongrakhananon, V.; Rojanasakul, Y.; Chanvorachote, P. Caveolin-1 Regulates Mcl-1 Stability and Anoikis in Lung Carcinoma Cells. Am. J. Physiol. Cell Physiol. 2012, 302, 1284–1292.
- Busaranon, K.; Plaimee, P.; Sritularak, B.; Chanvorachote, P. Moscatilin inhibits epithelial-to-mesenchymal transition and sensitizes anoikis in human lung cancer H460 cells. J. Nat. Med. 2016, 70, 18–27.
- Pramchu-em, C.; Meksawan, K.; Chanvorachote, P. Zinc sensitizes lung cancer cells to anoikis through down-regulation of Akt and caveolin-1. Nutr. Cancer 2016, 68, 1–8.
- Joo, J.C.; Hwang, J.H.; Jo, E.; Kim, Y.R.; Kim, D.J.; Lee, K.B.; Park, S.J.; Jang, I.S. Cordycepin induces apoptosis by caveolin-1-mediated JNK regulation of Foxo3a in human lung adenocarcinoma. Oncotarget 2017, 8, 12211–12224.
- Ecoy, G.A.U.; Chamni, S.; Suwanborirux, K.; Chanvorachote, P.; Chaotham, C. Jorunnamycin A from Xestospongia sp. Suppresses Epithelial to Mesenchymal Transition and Sensitizes Anoikis in Human Lung Cancer Cells. J. Nat. Prod. 2019, 82, 1861–1873.
- Wu, C.H.; Liu, F.C.; Pan, C.H.; Lai, M.T.; Lan, S.J.; Wu, C.H.; Sheu, M.J. Suppression of cell growth, migration and drug resistance by ethanolic extract of antrodia cinnamomea in human lung cancer A549 cells and C57BL/6J allograft tumor model. Int. J. Mol. Sci. 2018, 19, 791.
- Pengpaeng, P.; Sritularak, B.; Chanvorachote, P. Dendrofalconerol A sensitizes anoikis and inhibits migration in lung cancer cells. J. Nat. Med. 2015, 69, 178–190.
