Verticillium dahliae is a soil-borne plant pathogenic fungus that causes Verticillium wilt on hundreds of dicotyledonous plant species. V. dahliae is considered an asexually (clonal) reproducing fungus, although both mating type idiomorphs (MAT1-1 and MAT1-2) are present, and is heterothallic. Most of the available information on V. dahliae strains, including their biology, pathology, and genomics comes from studies on isolates with the MAT1-2 idiomorph, and thus little information is available on the MAT1-1 V. dahliae strains in the literature. We therefore evaluated the growth responses of MAT1-1 and MAT1-2 V. dahliae strains to various stimuli. Growth rates and melanin production in response to increased temperature, alkaline pH, light, and H2O2 stress were higher in the MAT1-2 strains than in the MAT1-1 strains. In addition, the MAT1-2 strains showed an enhanced ability to degrade complex polysaccharides, especially starch, pectin, and cellulose. Furthermore, several MAT1-2 strains from both potato and sunflower showed increased virulence on their original hosts, relative to their MAT1-1 counterparts. Thus, compared to MAT1-1 strains, MAT1-2 strains derive their potentially greater fitness from an increased capacity to adapt to their environment and exhibit higher virulence. These competitive advantages might explain the current abundance of MAT1-2 strains relative to MAT1-1 strains in the agricultural and sylvicultural ecosystems, and this study provides the baseline information on the two mating idiomorphs to study sexual reproduction in V. dahliae under natural and laboratory conditions.
1. Introduction
In most fungi, the mating-type locus represents a relatively small region of the genome, less than a few thousand base pairs, encoding transcription factors that act as master regulators of sexual reproduction [1]. One idiomorph of the mating-type locus contains a critical gene that encodes an α domain (MAT1-1-1), while the other contains a gene that encodes a DNA-binding domain of the high-mobility group (HMG) (MAT1-2-1) [2]. In heterothallic species, sexual recombination occurs only between partners of opposite mating types; and in contrast, homothallic species normally contain both MAT loci, which can either be located at a single MAT locus or on different chromosomes [3][4]. However, the regulatory impact of MAT-encoded transcription factors and MAT gene expression alone does not necessarily indicate sexual potential. In asexual fungi, MAT genes likely possess other important functions beyond mating. In the asexual Aspergillus oryzae, MAT1-1 and MAT1-2 specifically regulate over 1000 genes, including many with unknown functions [5]. In Penicillium chrysogenum, in addition to being involved in sexual development, MAT-controlled processes include asexual development, pallet morphology, polar hyphal growth, conidiospore germination, and secondary metabolite synthesis such as penicillin [6]. In most fungi, the mating-type locus represents a relatively small region of the genome, less than a few thousand base pairs, encoding transcription factors that act as master regulators of sexual reproduction [22]. One idiomorph of the mating-type locus contains a critical gene that encodes an α domain (MAT1-1-1), while the other contains a gene that encodes a DNA-binding domain of the high-mobility group (HMG) (MAT1-2-1) [23]. In heterothallic species, sexual recombination occurs only between partners of opposite mating types; and in contrast, homothallic species normally contain both MAT loci, which can either be located at a single MAT locus or on different chromosomes [12,24]. However, the regulatory impact of MAT-encoded transcription factors and MAT gene expression alone does not necessarily indicate sexual potential. In asexual fungi, MAT genes likely possess other important functions beyond mating. In the asexual Aspergillus oryzae, MAT1-1 and MAT1-2 specifically regulate over 1000 genes, including many with unknown functions [25]. In Penicillium chrysogenum, in addition to being involved in sexual development, MAT-controlled processes include asexual development, pallet morphology, polar hyphal growth, conidiospore germination, and secondary metabolite synthesis such as penicillin [26].
V. dahliae is a soil-borne, broad host-range plant pathogen that invades the xylem of susceptible plant species to cause vascular wilts [7][8]. Hundreds of dicotyledonous plants are hosts of V. dahliae, including many economically important crops such as lettuce, cotton, strawberries, and tomato [7][9][10][11]. V. dahliae is generally considered solely asexual (clonal), because the sexual cycle has never been documented either in nature or in the laboratory. The mating type idiomorph distribution in nature is skewed overwhelmingly toward MAT1-2, and extensive chromosomal rearrangements among different V. dahliae genomes could potentially interfere with meiosis. Moreover, the population structure of global V. dahliae strains is highly clonal [12][13][14][15]. This clonal structure is further complicated by abundant population subdivisions, including six main vegetative compatibility groups (VCGs) [16][17][18], races 1 (R1) and 2 (R2) that respond to host resistance genes in tomato and lettuce [19][20], a race 3 (R3) defined only in tomato [21], and two pathotypes, defoliating (D) and non-defoliating (ND) based on the presence or absence of defoliation caused by individual strains [22]. Some evidence supports a well-conserved ancestral or cryptic sexual reproduction in V. dahliae, including the two mating types in V. dahliae, and constitutively expressed sex-related genes [15]. Therefore, even though the evidence in the literature supports the clonal expansion of V. dahliae, the preservation of the machinery for sexual reproduction and the cryptic and ancestral sexual cycle in V. dahliae suggest that the potential for sexual reproduction exists in the fungus. V. dahliae is a soil-borne, broad host-range plant pathogen that invades the xylem of susceptible plant species to cause vascular wilts [27,28]. Hundreds of dicotyledonous plants are hosts of V. dahliae, including many economically important crops such as lettuce, cotton, strawberries, and tomato [27,29,30,31]. V. dahliae is generally considered solely asexual (clonal), because the sexual cycle has never been documented either in nature or in the laboratory. The mating type idiomorph distribution in nature is skewed overwhelmingly toward MAT1-2, and extensive chromosomal rearrangements among different V. dahliae genomes could potentially interfere with meiosis. Moreover, the population structure of global V. dahliae strains is highly clonal [32,33,34,35]. This clonal structure is further complicated by abundant population subdivisions, including six main vegetative compatibility groups (VCGs) [36,37,38], races 1 (R1) and 2 (R2) that respond to host resistance genes in tomato and lettuce [39,40], a race 3 (R3) defined only in tomato [41], and two pathotypes, defoliating (D) and non-defoliating (ND) based on the presence or absence of defoliation caused by individual strains [42]. Some evidence supports a well-conserved ancestral or cryptic sexual reproduction in V. dahliae, including the two mating types in V. dahliae, and constitutively expressed sex-related genes [35]. Therefore, even though the evidence in the literature supports the clonal expansion of V. dahliae, the preservation of the machinery for sexual reproduction and the cryptic and ancestral sexual cycle in V. dahliae suggest that the potential for sexual reproduction exists in the fungus.
Most of the available information on V. dahliae strains, including the biology, pathology, genetics, and genomics, is derived from studies on strains carrying the MAT1-2 idiomorph [23][24][25], and information on the MAT1-1 idiomorph is scarce. The lack of information on the MAT1-1 idiomorph has limited our understanding and research into the sexual mode of reproduction in V. dahliae. In recent years, V. dahliae strains with the MAT1-1 idiomorph have been frequently recovered from potato and sunflower in the Inner Mongolia Autonomous Region of China, and the distribution of the two mating-type strains is close to 1:1, which will significantly increase the temporal and spatial proximity of two V. dahliae mating-type strains to potentially enable mating and increase the risk of sexual recombination. A more efficient genome evolution that results in a novel progeny may ensue. Therefore, a comparative analysis of the basic growth and pathogenic characteristics of strains carrying the two mating-type idiomorphs will provide important data for the genetic analysis of the two idiomorphs and to evaluate the possibility of sexual reproduction in V. dahliae. Most of the available information on V. dahliae strains, including the biology, pathology, genetics, and genomics, is derived from studies on strains carrying the MAT1-2 idiomorph [43,44,45], and information on the MAT1-1 idiomorph is scarce. The lack of information on the MAT1-1 idiomorph has limited our understanding and research into the sexual mode of reproduction in V. dahliae. In recent years, V. dahliae strains with the MAT1-1 idiomorph have been frequently recovered from potato and sunflower in the Inner Mongolia Autonomous Region of China, and the distribution of the two mating-type strains is close to 1:1, which will significantly increase the temporal and spatial proximity of two V. dahliae mating-type strains to potentially enable mating and increase the risk of sexual recombination. A more efficient genome evolution that results in a novel progeny may ensue. Therefore, a comparative analysis of the basic growth and pathogenic characteristics of strains carrying the two mating-type idiomorphs will provide important data for the genetic analysis of the two idiomorphs and to evaluate the possibility of sexual reproduction in V. dahliae.
2. Characteristics of V. dahliae MAT1-1 Strain P48 and MAT1-2 Strain P50
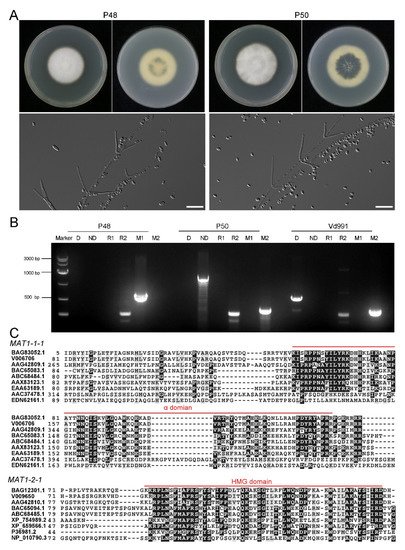
To obtain comparative information on the MAT1-1 and MAT1-2 strains, V. dahliae strain P48 with MAT1-1 and P50 with MAT1-2 isolated from potato in Inner Mongolia, China, were selected. The upper sides of the P48 and P50 cultures displayed white dense mycelia on the PDA medium that progressively darkened on the undersurface of the cultures as the melanized microsclerotia were produced, at about 7 days after incubation (
2. Characteristics of V. dahliae MAT1-1 Strain P48 and MAT1-2 Strain P50
To obtain comparative information on the MAT1-1 and MAT1-2 strains, V. dahliae strain P48 with MAT1-1 and P50 with MAT1-2 isolated from potato in Inner Mongolia, China, were selected. The upper sides of the P48 and P50 cultures displayed white dense mycelia on the PDA medium that progressively darkened on the undersurface of the cultures as the melanized microsclerotia were produced, at about 7 days after incubation (
A). During this interval, strain P50 produced more melanin than P48 (
A). The conidia from both strains were hyaline, elongated in clusters on phialides borne in whorls on branched aerial hyphae (
Figure 1A), typical characteristics of V. dahliae [26].
A), typical characteristics of V. dahliae [46]. Morphology and genetic characterization of the Verticillium dahliae strains P48 and P50. (
) Colony phenotype and conidial morphology of V. dahliae stains P48 and P50 on PDA medium. (
) Results of molecular identification of Verticillium dahliae strains P48 and P50 using primers specific for MAT1-1 (M1)/MAT1-2 (M2), race 1 (R1)/race 2 (R2), and defoliating (D)/non-defoliating (ND) characteristics. (
C) α and HMG domain comparison of sex-related transcriptional regulation factors MAT1-1-1 and MAT1-2-1 in P48 and P50 with other sexual fungi. BAG83052.1 and BAG12301.1: Verticillium dahliae VdLs.17; V006706 and V009650: Verticillium dahliae P48; AAG42809.1 and AAG42810.1: Fusarium graminearum; BAC65083.1 and BAC65094.1: Magnaporthe grisea; ABC68484.1 and ABC68485.1: Penicillium marneffei; AAX83123.1 and XP_754989.2: Aspergillus fumigatus; EAA63189.1 and XP_659566.1: Aspergillus nidulans; AAC37478.1 and P36981.2: Neurospora crassa; EDN62161.1 and NP_010790.3: Saccharomyces cerevisiae.
A number of PCR primers have been designed to amplify loci or markers associated with race (D or ND phenotype) and mating type idiomorphs [15][26][27][28]. The PCR assays indicated that strain P48 had the MAT1-1 marker and P50 the MAT1-2 marker, confirming that P48 and P50 represented strains with MAT1-1 and MAT1-2 idiomorphs, respectively ( ) α and HMG domain comparison of sex-related transcriptional regulation factors MAT1-1-1 and MAT1-2-1 in P48 and P50 with other sexual fungi. BAG83052.1 and BAG12301.1: Verticillium dahliae VdLs.17; V006706 and V009650: Verticillium dahliae P48; AAG42809.1 and AAG42810.1: Fusarium graminearum; BAC65083.1 and BAC65094.1: Magnaporthe grisea; ABC68484.1 and ABC68485.1: Penicillium marneffei; AAX83123.1 and XP_754989.2: Aspergillus fumigatus; EAA63189.1 and XP_659566.1: Aspergillus nidulans; AAC37478.1 and P36981.2: Neurospora crassa; EDN62161.1 and NP_010790.3: Saccharomyces cerevisiae.
A number of PCR primers have been designed to amplify loci or markers associated with race (D or ND phenotype) and mating type idiomorphs [35,46,47,48]. The PCR assays indicated that strain P48 had the MAT1-1 marker and P50 the MAT1-2 marker, confirming that P48 and P50 represented strains with MAT1-1 and MAT1-2 idiomorphs, respectively ( Figure 1B). Strain P50 was also characterized as the ND pathotype and belonged to race 2, while strain P48 contained the race 2 marker, and it neither carried the D nor the ND marker, suggesting that both P48 and P50 were race 2 strains and that P50 likely is not able to cause the defoliating phenotype on hosts such as olive, cotton, and okra. The well-characterized, highly virulent defoliating strain Vd991 represents the D pathotype, belongs to race 2, and carries the MAT1-2 marker. It was used as a control in these experiments.
To detect the sequence conservation and variability of the two mating-type master regulatory genes in strains P48 and P50, the coding sequences of the MAT1-1-1 and MAT1-2-1 genes from these two strains were aligned with the corresponding genes from eight other sexually reproducing fungi, two of which were homothallic (Fusarium graminearum, Aspergillus nidulans) and six were heterothallic (Magnaporthe grisea, Aspergillus fumigatus, Neurospora crassa, Saccharomyces cerevisiae, Penicillium marneffei), along with those of the V. dahliae strain VdLs.17, which has been used as the reference genome in many studies [23]. The results showed that both P48 and P50 had the conserved α or HMG domains similar to other sexual fungi, and most amino acids in the α or HMG domains were also highly conserved ( B). Strain P50 was also characterized as the ND pathotype and belonged to race 2, while strain P48 contained the race 2 marker, and it neither carried the D nor the ND marker, suggesting that both P48 and P50 were race 2 strains and that P50 likely is not able to cause the defoliating phenotype on hosts such as olive, cotton, and okra. The well-characterized, highly virulent defoliating strain Vd991 represents the D pathotype, belongs to race 2, and carries the MAT1-2 marker. It was used as a control in these experiments.
To detect the sequence conservation and variability of the two mating-type master regulatory genes in strains P48 and P50, the coding sequences of the MAT1-1-1 and MAT1-2-1 genes from these two strains were aligned with the corresponding genes from eight other sexually reproducing fungi, two of which were homothallic (Fusarium graminearum, Aspergillus nidulans) and six were heterothallic (Magnaporthe grisea, Aspergillus fumigatus, Neurospora crassa, Saccharomyces cerevisiae, Penicillium marneffei), along with those of the V. dahliae strain VdLs.17, which has been used as the reference genome in many studies [43]. The results showed that both P48 and P50 had the conserved α or HMG domains similar to other sexual fungi, and most amino acids in the α or HMG domains were also highly conserved ( Figure 1C).
3. Growth of MAT1-1 and MAT1-2 Strains under Different Culture Conditions
The growth characteristics of the MAT1-1 strain P48 were compared with those of the MAT1-2 strain P50 in response to a range of temperatures, pH treatments, light/dark regimes, carbon sources, and stress conditions. Furthermore, the growth of five additional isolates of MAT1-1 (S2, S11, S109, P51, and P56) and MAT1-2 (S1, S12, S23, P52, and P90) strains from sunflower (S) and potato (P) was also determined to ensure that the results obtained with P48 and P50 were not unique to these strains. Detailed information on these strains is provided in Table S1.
C).
3. Growth of MAT1-1 and MAT1-2 Strains under Different Culture Conditions
The growth characteristics of the MAT1-1 strain P48 were compared with those of the MAT1-2 strain P50 in response to a range of temperatures, pH treatments, light/dark regimes, carbon sources, and stress conditions. Furthermore, the growth of five additional isolates of MAT1-1 (S2, S11, S109, P51, and P56) and MAT1-2 (S1, S12, S23, P52, and P90) strains from sunflower (S) and potato (P) was also determined to ensure that the results obtained with P48 and P50 were not unique to these strains. Detailed information on these strains is provided in Table S1. 3.1. Temperature
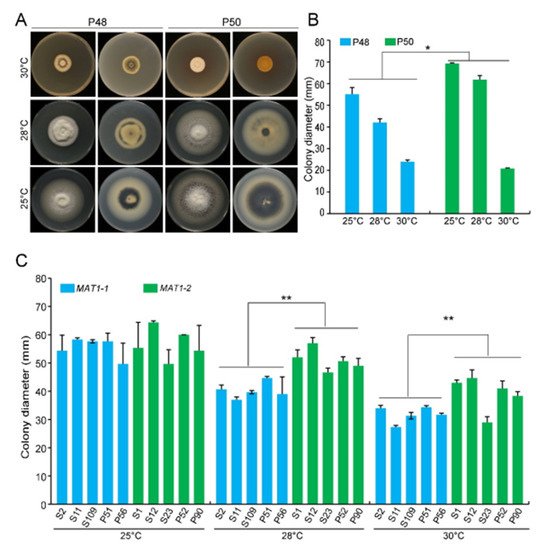
V. dahliae has an optimal temperature range of 22−27 °C, and limited growth occurs above 32 °C [29]. The MAT1-1 strain P48 produced a predominantly white mycelium, but the MAT1-2 strain P50 produced less mycelium, displayed faster colony growth, and accumulated higher amounts of melanin following incubation for 15 days at 25 °C (
V. dahliae has an optimal temperature range of 22−27 °C, and limited growth occurs above 32 °C [49]. The MAT1-1 strain P48 produced a predominantly white mycelium, but the MAT1-2 strain P50 produced less mycelium, displayed faster colony growth, and accumulated higher amounts of melanin following incubation for 15 days at 25 °C ( MAT1-1 and MAT1-2 strains of Verticillium dahliae grown under temperature stress conditions. (
) Growth phenotype of P48 and P50 on PDA medium at 25 °C, 28 °C, and 30 °C after culturing for 15 days. (
) Colony diameter of strains P48 and P50 on PDA medium at 25 °C, 28 °C, and 30 °C after culturing for 15 days. (
) Colony diameter of MAT1-1 and MAT1-2 strain collections on PDA medium at 25 °C, 28 °C, and 30 °C after culturing for 15 days. P and S indicate strains isolated from potato and sunflower, respectively. Asterisks * and ** indicate significant differences; p < 0.05 and p < 0.01, respectively, according to an unpaired Student’s t-test.
The growth rates of both P50 and P48 were significantly lower at 28 °C and 30 °C relative to 25 °C (
A). However, even at 28 °C, the colony diameter of the MAT1-2 strain P50 was significantly larger than that of the MAT1-1 strain P48 (
A,B). At 30 °C, the growth of both strains was restricted and was not significantly different (
A,B). These results suggest that temperatures <28 °C had fewer influences on the MAT1-2 strain P50 than on the MAT1-1 strain P48, but once the temperature exceeded 30 °C, the growth of both strains was nearly arrested.
Additional MAT1-1 and MAT1-2 V. dahliae strains, under the optimum temperature (25 °C), showed no detectable differences in their growth rates for up to 15 days incubation, except that the MAT1-1 strains mainly developed white mycelia with less melanin accumulation than the MAT1-2 strains (
C and Figure S1). At 28 °C and 30 °C, the diameters of the MAT1-2 strains were significantly higher than those of the MAT1-1 strains ( Figure 2C and Figure S1). Higher temperatures therefore restrict growth and melanin production in both MAT1-1 and MAT1-2 strains, but these effects were more pronounced in the MAT1-1 strains than in the MAT1-2 strains.
C and Figure S1). Higher temperatures therefore restrict growth and melanin production in both MAT1-1 and MAT1-2 strains, but these effects were more pronounced in the MAT1-1 strains than in the MAT1-2 strains. 3.2. pH
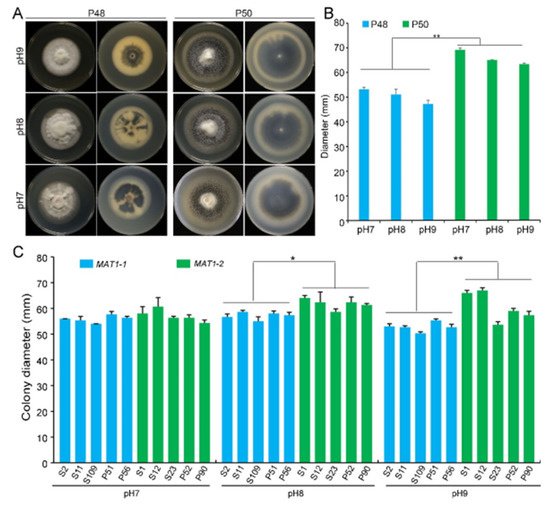
Verticillium wilt of cotton normally occurs in near-neutral to alkaline soils at a pH in the range 6–9. At pH 5.5 or below, the growth, microsclerotia production, and survival of V. dahliae are generally inhibited [29]. Under laboratory conditions, the optimum pH for V. dahliae on the PDA medium was approximately 6.5. To determine the differences in the growth of the two idiomorphs, three PDA plates at pH 7.0, 8.0, and 9.0 each were incubated at 25 °C. Strain P50 produced more melanin under these pH values than at pH 6.5 (
Verticillium wilt of cotton normally occurs in near-neutral to alkaline soils at a pH in the range 6–9. At pH 5.5 or below, the growth, microsclerotia production, and survival of V. dahliae are generally inhibited [49]. Under laboratory conditions, the optimum pH for V. dahliae on the PDA medium was approximately 6.5. To determine the differences in the growth of the two idiomorphs, three PDA plates at pH 7.0, 8.0, and 9.0 each were incubated at 25 °C. Strain P50 produced more melanin under these pH values than at pH 6.5 ( A). Melanin accumulation increased with increasing pH, with the maximum melanin enrichment occurring at pH 9.0 (
A). However, the melanin production by the MAT1-1 strain P48 showed no obvious change with the pH (
A). Increasing pH did not alter the growth rates of either strain, but at each pH tested, P50 exhibited a higher colony growth than strain P48 (
A,B). Alkaline pH stimulated the accumulation of melanin by the two mating-type strains, but had no quantifiable influence on colony growth rates. The influence of pH on the growth of MAT1-1 and MAT1-2 strains of Verticillium dahliae. (
) Growth phenotype of P48 and P50 at pH 7, pH 8, and pH 9 after culturing for 15 days. (
) Colony diameters of P48 and P50 at pH 7, pH 8, and pH 9 after culturing for 15 days. (
) Colony diameter of MAT1-1 and MAT1-2 strain populations at pH 7, pH 8, and pH 9 after culturing for 15 days. P and S indicate strains isolated from potato and sunflower, respectively. Asterisks * and ** indicate significant differences; p < 0.05 and p < 0.01, respectively, according to an unpaired Student’s t-test.
The growth rates of the five additional MAT1-1 strains were not altered by the pH, but melanin accumulation was reduced at pH 8 and 9, regardless of whether they were isolated from potato or sunflower. In contrast, MAT1-2 strains from potato and sunflower displayed higher growth rates at higher pH, and melanin production was relatively unaffected compared to the MAT1-1 strains (
Figure 3C and Figure S2). Overall, relative to the MAT1-1 strains, MAT1-2 strains not only grew faster but also accumulated more melanin under near-neutral and alkaline pH.
C and Figure S2). Overall, relative to the MAT1-1 strains, MAT1-2 strains not only grew faster but also accumulated more melanin under near-neutral and alkaline pH. 3.4. Light/Dark
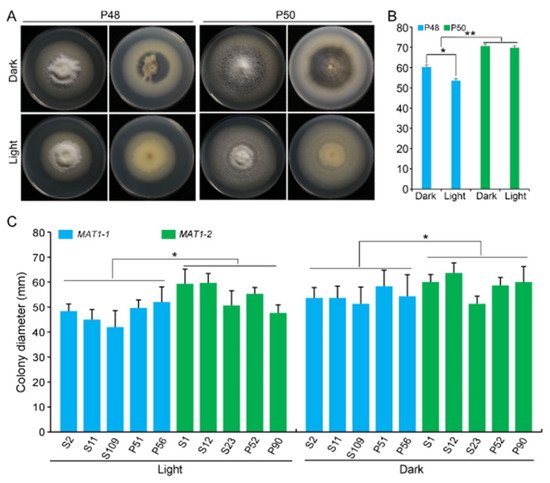
The growth of strains P48 and P50 was evaluated on a PDA medium at 25 °C under dark and light conditions. Under the dark conditions examined, both the P48 and P50 strains produced melanin, although the growth rates and melanin accumulation in strain P50 were higher relative to those of P48 (
A). However, under the light conditions examined, neither P48 nor P50 produced visible melanin, with both forming only white mycelia (
A). The colony diameter of P48 was smaller under light conditions than under dark conditions, but the colony diameters of the P50 strain showed no obvious differences between light and dark conditions (
B). Under the light or dark conditions, the growth rates of P50 were higher than those of P48 (
B). These results suggested that light interfered with the melanin production in both strains and limited the rate of growth of the MAT1-1 strain P48 but did not influence the growth rate of the MAT1-2 strain P50. The effect of light and dark on MAT1-1 and MAT1-2 strains of Verticillium dahliae. (
) Growth phenotype of P48 and P50 under light and dark conditions after culturing for 15 days. (
) Colony diameters of P48 and P50 under light and dark conditions after culturing for 15 days. (
) Colony diameter of MAT1-1 and MAT1-2 strain populations under light and dark conditions after culturing for 15 days. P and S indicate strains isolated from potato and sunflower, respectively. Asterisks * and ** indicate significant differences; p < 0.05 and p < 0.01, respectively, according to an unpaired Student’s t-test.
The influence of light was further examined on the growth of five additional strains from MAT1-1 and MAT1-2. Under the light conditions tested, there was no visible melanin production in the MAT1-1 strains, with the exception of S2 (Figure S3). Similarly, melanin accumulation in the MAT1-2 strains was also minimal under light, with only strains S23 and P52 producing small amounts of melanin (Figure S3). The growth rate of MAT1-2 strains whether under dark or light conditions was higher relative to that of the MAT1-1 strains; and MAT1-2 strains produced more melanin under the dark conditions than the MAT1-1 strains ( Figure 4C and Figure S3). These results indicate that light restricted melanin production in both mating-types but had relatively less influence on the growth rate of MAT1-2 strains versus MAT1-1 strains.
3.4. Stress Tolerance (Osmotic Stress, Oxidative Stress, Cell Wall Integrity Stress)
To evaluate the stress tolerance of the two mating type strains, their growth on media that imposed osmotic stress (sorbitol-containing medium at three concentrations: 1, 1.5, and 2 mol/L), oxidative stress (H2O2-containing medium at three concentrations: 0.9, 1.2, and 1.8 mmol/L), and cell wall integrity stress (Congo red-containing medium at three concentrations: 100, 200, and 300 μg/mL) was evaluated.
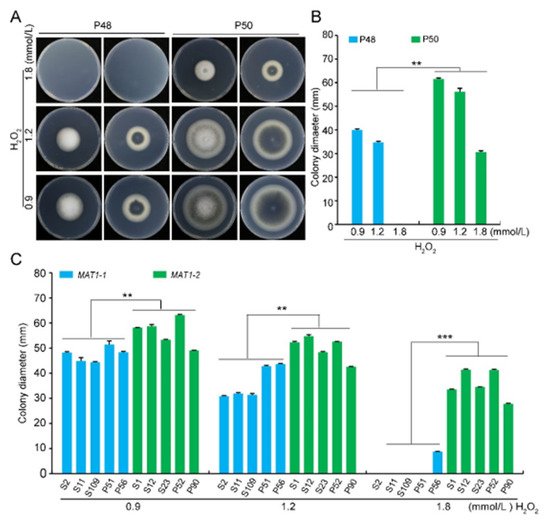
Under osmotic stress, the growth rate of both P48 and P50 decreased as the sorbitol concentration increased, and there was no apparent melanin production under sorbitol stress. While there were no differences in the growth rates between the two strains under sorbitol concentrations of 1 and 1.5 mol/L, the growth rate of the P50 strain was higher relative to that of strain P48 at 2 mol/L sorbitol (Figure S5A,B). Similarly, under cell wall integrity stress, the growth rates of both P48 and P50 decreased as the Congo red concentration increased (Figure S5C,D). Strain P50 produced more melanin at 100 and 200 μg/L Congo red than P48 (Figure S5C,D). Under the cell wall stress conditions tested, there were also no apparent differences in growth rate between the two strains (Figure S5D). The growth rates of both strains decreased as the oxidative stress from H2O2 concentration increased. However, there was almost no growth of strain P48 at the concentration of 1.8 mmol/L H2O2 ( C and Figure S3). These results indicate that light restricted melanin production in both mating-types but had relatively less influence on the growth rate of MAT1-2 strains versus MAT1-1 strains.
2.3. Stress Tolerance (Osmotic Stress, Oxidative Stress, Cell Wall Integrity Stress)
To evaluate the stress tolerance of the two mating type strains, their growth on media that imposed osmotic stress (sorbitol-containing medium at three concentrations: 1, 1.5, and 2 mol/L), oxidative stress (H2O2-containing medium at three concentrations: 0.9, 1.2, and 1.8 mmol/L), and cell wall integrity stress (Congo red-containing medium at three concentrations: 100, 200, and 300 μg/mL) was evaluated.
Under osmotic stress, the growth rate of both P48 and P50 decreased as the sorbitol concentration increased, and there was no apparent melanin production under sorbitol stress. While there were no differences in the growth rates between the two strains under sorbitol concentrations of 1 and 1.5 mol/L, the growth rate of the P50 strain was higher relative to that of strain P48 at 2 mol/L sorbitol (Figure S5A,B). Similarly, under cell wall integrity stress, the growth rates of both P48 and P50 decreased as the Congo red concentration increased (Figure S5C,D). Strain P50 produced more melanin at 100 and 200 μg/L Congo red than P48 (Figure S5C,D). Under the cell wall stress conditions tested, there were also no apparent differences in growth rate between the two strains (Figure S5D). The growth rates of both strains decreased as the oxidative stress from H2O2 concentration increased. However, there was almost no growth of strain P48 at the concentration of 1.8 mmol/L H2O2 ( A,B). Unlike the other stress conditions examined, the growth rate of the MAT1-2 strain P50 was higher than that of the MAT1-1 strain P48 in response to H2O2, indicating that the MAT1-2 strain P50 had a greater resistance to oxidative stress than the MAT1-1 strain P48. Growth of MAT1-1 and MAT1-2 strains of Verticillium dahliae in oxidative stress conditions. (
) Growth phenotype of P48 and P50 in response to serial concentrations of H2O2 (0.9, 1.2, 1.8 mmol/L). (
) Colony diameters of strains P48 and P50 after culturing for 15 days with different concentrations of H2O2 (0.9, 1.2, 1.8 mmol/L). (
C) Colony diameters of MAT1-1 and MAT1-2 populations in response to serial concentrations of H2O2 (0.9, 1.2, 1.8 mmol/L) at 15 days of growth. P and S indicate strains isolated from potato and sunflower, respectively. Asterisks ** and *** indicate significant differences; p < 0.01 and p < 0.001, respectively, according to an unpaired Student’s t-test.
Colony diameters of the additional MAT1-1 strains evaluated were significantly smaller in size as the H2O2 concentration increased. At 1.8 mmol/L H2O2 none of the MAT1-1 strains grew except P56, which produced very small colonies (Figure S6). The growth rates of the MAT1-2 strains were also slower as the H2O2 concentrations increased, the only difference being that the colony diameters were larger relative to those observed in the MAT1-1 strains
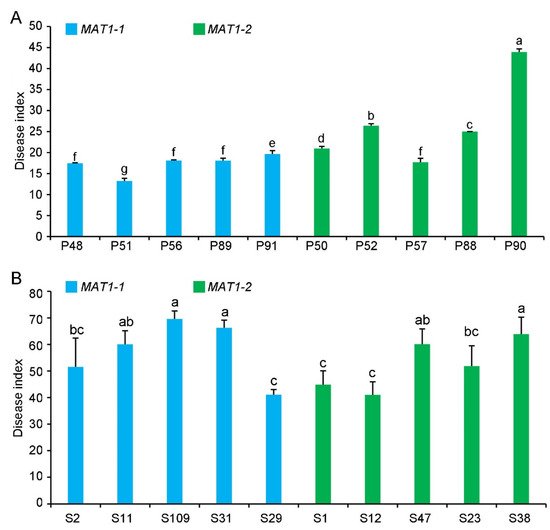
4. Pathogenicity of MAT1-1 and MAT1-2 Strains on Their Original Hosts
The virulence of the MAT1-1 and MAT1-2 strains was evaluated on potato or sunflower. Most of the MAT1-2 strains from potato were more virulent than the MAT1-1 strains on potato, except for strain P57 ( ) Colony diameters of MAT1-1 and MAT1-2 populations in response to serial concentrations of H2O2 (0.9, 1.2, 1.8 mmol/L) at 15 days of growth. P and S indicate strains isolated from potato and sunflower, respectively. Asterisks ** and *** indicate significant differences; p < 0.01 and p < 0.001, respectively, according to an unpaired Student’s t-test.
Colony diameters of the additional MAT1-1 strains evaluated were significantly smaller in size as the H2O2 concentration increased. At 1.8 mmol/L H2O2 none of the MAT1-1 strains grew except P56, which produced very small colonies (Figure S6). The growth rates of the MAT1-2 strains were also slower as the H2O2 concentrations increased, the only difference being that the colony diameters were larger relative to those observed in the MAT1-1 strains
4. Pathogenicity of MAT1-1 and MAT1-2 Strains on Their Original Hosts
The virulence of the MAT1-1 and MAT1-2 strains was evaluated on potato or sunflower. Most of the MAT1-2 strains from potato were more virulent than the MAT1-1 strains on potato, except for strain P57 (
). However, the overall virulence of MAT1-2 and MAT1-1 strains from sunflower was not significantly different on sunflower (
Pathogenicity of MAT1-1 and MAT1-2 strain populations of Verticillium dahliae on potato and sunflower. (
) Disease index of MAT1-1 and MAT1-2 strain populations isolated from potato (P) (MAT1-1 strains: P48, P51, P56, P89, P91; MAT1-2 strains: P50, P52, P57, P88, and P90) three weeks after inoculating potato (He 15). (
B) Disease index of MAT1-1 and MAT1-2 strains isolated from sunflower (LD 5009) (S) (MAT1-1 strains: S2, S11, S109, S31, and S29; MAT1-2 strains: S1, S12, S47, S23, and S38) three weeks after the inoculation of sunflower. Columns with different letters represent a statistical significance of p < 0.05 according to Duncan’s new multiple range test.
) Disease index of MAT1-1 and MAT1-2 strains isolated from sunflower (LD 5009) (S) (MAT1-1 strains: S2, S11, S109, S31, and S29; MAT1-2 strains: S1, S12, S47, S23, and S38) three weeks after the inoculation of sunflower. Columns with different letters represent a statistical significance of p < 0.05 according to Duncan’s new multiple range test.