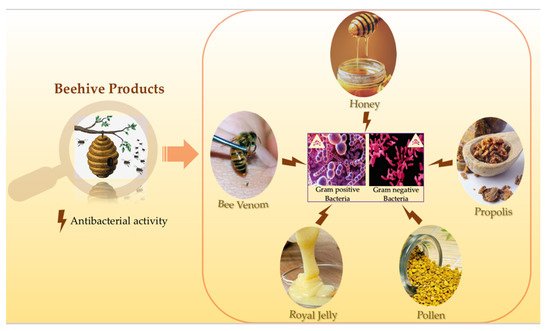
Apitherapy is a branch of unconventional medicine that relies on the usage of bee products which consist of honey, pollen, propolis, royal jelly and bee venom (BV). Besides having high nutritional importance and health benefits, honey showed antifungal, antiviral, antiseptic, anticancer, anti-diabetic, anti-inflammatory and cardio protective activities. As for the BV, despite its possible adverse effects like the allergic reactions that might occur after the bee sting, one cannot disregard its various therapeutic effects. BV exerts anticancer, antiviral, antifungal and antibacterial effects, it is also used for the treatment of many neurodegenerative diseases. Regarding propolis, it has the capacity to fight against cancer and many microorganisms. Moreover, pollen possesses antioxidant, anticoagulant and anti-inflammatory properties. Also, royal jelly exhibits several interesting biological activities including antioxidant, anti-aging, antitumor anti-inflammatory, antimicrobial, and neurotrophic activities. Hence, this review aims to highlight one of the most important and commonly shared biological activity of all of the above-mentioned beehive products, which is the antibacterial activity.
- antibacterial activity
- honeybee products
- honey
- bee venom
- propolis
- royal jelly
- pollen
1. Overview
Honeybees are one of the most marvelous and economically beneficial insects. As pollinators, they play a vital role in every aspect of the ecosystem. Beehive products have been used for thousands of years in many cultures for the treatment of various diseases. Their healing properties have been documented in many religious texts like the Noble Quran and the Holy Bible. Honey, bee venom, propolis, pollen and royal jelly all demonstrated a richness in their bioactive compounds which make them effective against a variety of bacterial strains. Furthermore, many studies showed that honey and bee venom work as powerful antibacterial agents against a wide range of bacteria including life-threatening bacteria. Several reports documented the biological activities of honeybee products but none of them emphasized on the antibacterial activity of all beehive products. Therefore, this review aims to highlight the antibacterial activity of honey, bee venom, propolis, pollen and royal jelly, that are produced by honeybees.
2. Honeybees
3. Antibacterial Activity of Bee Honey
4. Antibacterial Activity of Bee Venom
Bee venom (BV) is a widely recognized toxin secreted by female worker bee’s poison glands as a protection mechanism [75]. Although it is toxic to predators, BV has been used for many medicinal purposes since Ancient Egypt (4000 BC). In bee venom therapy (BVT), the toxin is applied directly or indirectly into the body for the treatment of certain diseases, such as rheumatism arthritis [76–78]. Venoms from different animals or organisms are considered as promising antimicrobial agents that work against several bacteriological pathogenesis [79]. The antimicrobial activity and medical use of BV is due to the presence of bioactive molecules in particular peptides which are the main components and consti‐ tute 48–50% of dry BV weight [80]. The Apis mellifera venom is an odorless and transparent liquid that is made up of 88% of water and only 0.1 μg of dry venom [78]. The dry venom itself is highly rich in peptides notably melittin, apamin, adolapin, and mast cell degran‐ ulating (MCD) peptide, enzymes such as phospholipase A2 (PLA2) and hyaluronidas [81,82]. Among these peptides, melittin represents the main biological active compound in bee venom. It represents about 50% of the dry BV [83]. Another major component is phospholipase A2, being the most toxic bee venom peptide [84]. These two components can act synergically and damage the cell membrane [8]. Melittin possesses a low selectivity to the cell membrane and acts strongly on its lipids via the process of pore formation. This process causes the release of the cell cytoplasmic contents and leads to cell lysis [85] . This mode of action is most likely to be responsible of the antibacterial properties of BV [86]. Several studies have demonstrated the ability of BV to kill both Gram‐negative and Gram‐ positive bacteria [87,88]. Indeed, the antibacterial activity of BV from Apis mellifera pure‐ bred as well as hybrid was tested against five bacterial strains. The results showed that BV displayed an antibacterial activity against all five bacterial strains. Other studies proved that BV can be used as a complementary antimicrobial agent against pathogenic bacteria even if it is collected by different methods (i.e., from the top of the frames or from under the frames with distance between the bottom board and the brood chamber) [89]. Additionally, the bacterium Borrelia burgdorferi is known to be the main cause of Lyme disease. Researchers found that both BV and melittin possess significant effects on all the morphological forms of B. burgdorferi, even on antibiotic resistant attached biofilms. How‐ ever, antibiotics when administrated alone or combined together had limited effects [90]. Food‐borne pathogens present a risk to develop antimicrobial resistance and generate biofilms such as Salmonella. Sixteen Salmonella strains isolated from poultry were tested against apitoxin (Bee venom). In 14 of the 16 tested strains, apitoxin reduced the biofilm formation and destroyed the preformed biofilm by 47%. Also, apitoxin increased the mo‐ bility of the bacteria and the MIC varied between 1024–256 μg/mL [7]. Aiming to find an alternative to antibiotics in the poultry industry, broiler chicks were sprayed with BV to evaluate its immunoprophylactic effects against Salmonella gallinarum. The results showed that BV increased the antibody production against formalin‐killed S. gallinarum [91].
Others previous studies focused on the antibacterial effect of bee venom against ad‐ ditional type of bacteria such as S. aureus and E. coli. The results proved BV to be active in killing both bacterial strains. At 10 × MIC, the regrowth of E. coli was not observed at 18 h while For S. aureus at 5 × MIC [92,93]. Regarding the MIC for Gram‐positive strains, the range was from 200 μg/mL to 8 μg/mL for the most sensitive species B. subtilis. Alterna‐ tively, Gram‐negative strains were found to be more resistant to BV (MIC 60 to >500 μg/mL). This can be explained by the nature of the bacterium cell membrane [87,93]; the outer membrane of Gram‐negative bacteria encompasses lipopolysaccharides (LPS) which obstruct the penetration of melittin presented in the BV. Thus, making melittin re‐ sponsible for the antibacterial activity. While in Gram‐positive bacteria, LPS are absent, indicating that melittin can penetrate the cell membrane more easily to form pores and increases the cell permeability through the cytoplasmic membrane [94]. PLA2, which is also presented in abundance in the crude BV can disrupt the cell membrane of Gram‐ negative bacteria indirectly by hydrolyzing phospholipids enzymatically. Other works re‐ ported the antibacterial activity of the crude BV and its two main biopeptides melittin and PLA2 against oral pathogen responsible of tooth decay: Lactobacillus casei, Streptococcus sal- ivarius, S. mutans, Staphylococcus mitis, Staphyslococcus sobrinus, Staphylococcus sanguinis and Enterococcus faecali. Melittin was twice as active as the crude BV against the tested bacteria (4 to 40 μg/mL). As for PLA2, it was only active against L. casei at >400 μg/mL [95,96]. Similarly, melittin showed antimicrobial activity against Staphylococcal strains as well as MRSA strains while PLA2 did not show any effect on the same strains [97]. Also, it has been demonstrated that certain amino acids and their positions play a crucial role in the antibacterial activity exerted by melittin. For example, the absence of a proline residue in position 14 significantly diminishes the antimicrobial activity of melittin [98]. Likewise, two synthetic melittins, one asparagine‐substituted melittin (Mel‐N) and the other serine‐ substituted melittin (Mel‐S) were able to penetrate the membrane of E. coli but the latter was more efficient [99]. Intriguingly, the antibacterial mechanism of new melittin derived‐ peptides MDP1 (GIGAVLKVLTTGLPALIKRKRQQ) and MDP2 (GIGAVLKWL‐ PALIKRKRQQ) was evaluated against multidrug resistant strains such as E. coli, P. aeru- ginosa and MRSA. The two derived peptides showed strong antibacterial activity against reference strains of the three multidrug resistant strains compared to the indigenous melittin via membrane alteration and damage [100].
On the other hand, antimicrobial treatments are becoming less efficient by the day due to the increasing number of multi‐drug resistant (MDR) bacteria as well as bacterial biofilms which are very hard to treat. Between 317 positive specimens for bacterial growth, 124 were MDR isolate of Gram‐negative and Gram‐positive bacteria. BV demon‐ strated a complete growth inhibition percentage (100%) against all tested MDR‐isolates with a wide range of MICs and MLCs concentration‐ranging between 3.125–50 μg/mL and 6.25–100 μg/mL, respectively against all MDR‐GNB and GPB one [101]. The antibacterial activity of BV was tested alone or in combination with antibiotics (ampicillin, gentamicin, penicillin, or vancomycin) on the growth of MRSA strains. A partial synergetic effect was indicated by the index of inhibitory concentration ranging from 0.631 to 1.002 for the com‐ bination of BV with ampicillin or penicillin which made the MRSA strains more suscepti‐ ble to the combination of BV with gentamicin or vancomycin rather than the combination of BV with ampicillin or penicillin [102]. BV was studied for its antibacterial activity against four MDR‐Acinetobacter baumannii bacterial strains. Results showed an inhibition of the bacterial growth of MDR‐A. baumannii strains with MIC value 31.25 mg/mL [103]. Melittin combined with doripenem caused a significant decrease in the MIC of MDR‐ A. baumannii strains. The combination of melittin–ceftazidime and melittin–doripenem was administrated to MDR‐P. aeruginosa strains. This caused a decrease in melittin dosage which led to a decrease in melittin cytotoxicity. Therefore, the combination of melittin– doripenem (for A. baumannii) and melittin–doripenem with melittin– ceftazidime (for P. aeruginosa) at their MIC dose might serve as a promising therapeutical treatment against MDR bacteria [104].
The investigation of BV in pre‐clinical and clinical application is still very slow de‐ spite its efficacity when combined with other drugs to treat different types of bacteria. Researchers found that BV and melittin combined with other antibiotics like vancomycin, oxacillin, and amikacin yielded fractional inhibitory concentration (FIC) indices fluctuat‐ ing between 0.24 and 0.5. Apitoxin and melittin tested against 51 strains of bacteria exhib‐ ited strong antibacterial activity against both Gram‐negative bacteria (MIC values be‐ tween 30 and >500 μg/mL) and Gram‐positive (MIC values between 10 and 100 μg/mL). Moreover, a strong anti‐MRSA and anti‐VRE activity was shown by BV and melittin at MIC values of 6–800 μg/mL [87]. When combined with oxacillin, BV and melittin showed an antibacterial activity against MRSA ATCC 3359 [84]. The antibacterial activity of melit‐ tin as well as its synergistic effect with β‐lactam antibiotics against A. baumannii was eval‐ uated by means of the broth microdilution method. The MIC value of melittin was 4 μg/mL and results showed inhibition of bacterial growth. Furthermore, FIC indices for combination of melittin with co‐amoxiclav, ceftazidime and imipenem showed a syner‐ getic effect [105].
All of the above‐ mentioned information confirm that BV and its two major com‐ pounds melittin and PLA2 exhibit a very promising antibacterial activity against several pathogenic bacterial strains even against multidrug resistant bacteria. However, one should always keep in mind that these biopeptides are venomous and might act as toxic agents if administrated the wrong way. Melittin is the most toxic component in BV. While being responsible for the majority of the pain associated with bee stings, it only induces a minor allergic reaction. Contrary to PLA2 which is the most allergenic and immunogenic protein in bee venom [106,107]. Bee envenomation can lead to many clinical manifesta‐ tions such as local inflammatory reactions, allergic reactions, anaphylactic shocks, and systemic toxic reactions [108]. The ideal treatments against the toxic effect of bee venom are corticosteroids, antihistamine, and intramuscular adrenaline depending on the sever‐ ity of the bee envenomation [109,110].
Many clinical studies on bee venom have shown promising results. Acne, one of the most common problems among teenagers, is usually treated with antibiotics to kill the causative bacteria but in some cases bacterial resistance can occur [111]. In a clinical study conducted by Han et al., skincare products with or without were tested on 12 subjects suffering from acne. The results showed a 57.7% decrease in ATP measured to evaluate the decrease in skin microbes. Thus, cosmetics and skincare products containing BV can used as a promising therapeutic anti‐acne agent [112].
5. Antibacterial Activity of Propolis
Among the many products of honeybees, propolis is considered as one of the most interesting products utilized as the main defensive element and building block in the hives. Propolis is used by bees to fill the cavity in the walls of the hive, it is also used to repair combs and strengthen its thin borders. On the other hand, propolis can mummify intruders that cannot be transported outside thus preventing their decay [113]. Since an‐ cient times, the anti‐putrefactive property has been used by humans, notably by Egyp‐ tians. The origin of propolis, also known as “bee glue”, had long been a subject of discus‐ sion, some thought it came from bees themselves while others thought it originated from plants. Nowadays, the approximate composition of propolis and the factors affecting it became clearer [114]. Propolis is now confirmed to be a bee product made from plants. Bees collect resinous vegetable matter from different parts of the plant such as lipophilic matter on leaves and their buds, latex as well as mucilage [115]. Also, the bees are able to cut the fragments of vegetative tissues in order to extract the products necessary for the production of propolis [116]. Furthermore, propolis is a complex mixture with a variable composition that depends on the geographical region and the plant species used in its production. Surprisingly, having different composition does not indicate different biolog‐ ical activities [12]. Propolis is a resin that can occur in different colors and exhibits a pleas‐ ant aromatic resin smell of great value. Propolis is mainly composed of resins, flavonoids, polyphenols, terpenoids, essential oils, and other organics and minerals [117] . The extrac‐ tion of propolis and its dissolution is needed in order to release its most active ingredients. The extraction process is also required for the later usage of propolis for medicinal pur‐ poses. It is also important to mention that solvent types used in extraction can affect the biological activity obtained from propolis. For the antibacterial activity, the abundancy of flavonoids and phenols plays a key role in conferring this bioactivity [118]. Additionally, the presence of many bioactive ingredients and in different concentrations is crucial in preventing the occurrence of bacterial resistance [119]. As for the mechanism of action, propolis can act directly on the microorganism or indirectly by triggering the immune system which results in the activation of the body natural defense mechanism. Studies showed that Gram‐negative bacteria are more resistant to propolis than Gram‐positive bacteria. This can be explained by two main factors, the first one being the specific struc‐ ture of the Gram negative bacteria membrane and the second one being the secretion of hydrolytic enzymes which destroy the active ingredients present in propolis [120].
Artepillin C (3, 5‐diprenyl‐p‐coumaric acid) is considered as one of the most im‐ portant phenolic compounds found in propolis. Propolis ethanolic extract showed higher antibacterial activity against MRSA in comparison with hexane extract due to the higher concentration of Artepillin C in ethanolic extract [121]. The antibacterial activity of ethanol extracted propolis and its derivative compounds was investigated against P. gingivalis, a bacterium responsible for periodontal diseases. Artepillin C showed a bacteriostatic effect with membrane blebbing [122]. Propolis contains other phenyl derivatives such as 2‐di‐ methyl‐8‐prenylchromene and 3‐prenylcinnamic acid allyl ester. After investigating the chemical constituents and the antibacterial activity of the ethanolic extract of propolis, the results showed a high concentration of p‐coumaric acid, artepillin‐C, drupanin and kaempferide alongside an antibacterial activity against Listeria monocytogenes, Enterococcus faecalis, S. aureus and Staphylococcus saprophyticus [123]. Also, propolis contains pino‐ cembrin and apigenin. Chilean propolis was subjected to a study and researchers found that the antibacterial activity of pinocembrin and apigenin is higher than that of polyphe‐ nols mixture or even chlorhexidine against S. aureus [124]. Numerous work reported the presence of antibacterial activity of pinocembrin against different bacterial strains such as S. mutans, S. sobrinus, S. aureus, E. faecalis, L. monocytogenes, P. aeruginosa and K. pneumoniae [125,126].
Nepalese ethanolic extract of propolis was evaluated for both its antimicrobial activ‐ ity and its chemical composition. The main components were flavonoid aglycones (mainly neoflavonoids, isoflavonoids) and pterocarpans. Nepalese propolis exhibited its highest antibacterial activity against H. pylori, S. aureus and Shigella flexneri. When combined with amikacin and tetracycline, the same propolis yielded the strongest effect against S. aureus [126]. The ethanolic extract of polish propolis (EEPP) was examined for its antibacterial activity against MRSA and MSSA. The average MIC was 0.54 mg/mL and EEPP was ef‐ fective against 12 S. aureus strains. Also, EEPP combined with 8 antistaphylococcal drugs, provided a stronger antibacterial effect against the tested strains than EEPP alone [127]. The geographical location affects the composition of propolis as well as its antimicrobial activity. 40 ethanol extract of propolis (EEP) collected worldwide were evaluated for their antibacterial activity against S. aureus strains. Results showed a moderate activity for Asian and African samples with MICS ranging from 0.0156 to >0.5 mg/mL and 0.0078 to >0.5 mg/mL, respectively with a similar results displayed by samples collected from North and South America and Europe [128]. EEP samples from three different regions of turkey were analyzed. MIC values varied and amounted to 0.018, 0.162, and 0.101 mg/mL and samples showed high antibacterial activity [129].
The number of studies concerning the antibacterial effect of propolis against anaero‐ bic bacteria remains limited. However, it has been presented that propolis is more active on Gram‐positive anaerobic bacteria than on Gram‐negative ones. Propolis showed anti‐ bacterial activity against Lactobacillus acidophilus, Prevotella oralis, P. gingivalis, Fusobacte- rium nucleatum, Peptostreptococcus anaerobius, Actinomyces naeslundii and Veillonella parvula [130]. Moreover, research shows an important activity of propolis against Porphyromona, Fusobacterium, Propionibacterium, Clostridium, Prevotella, Actinomyces and Bacteroides spe‐ cies [131,132].
Propolis displays different antibacterial mechanisms such as inhibition of cell divi‐ sion, collapse of membranes and cell walls of the microbial cytoplasm, decreasing bacte‐ rial motility, enzymatic inactivation, bacteriolysis and inhibition of protein synthesis [133]. All the different compounds that constitute the propolis composition are rich in polyphenols which work together with different microbial proteins to form both ionic and hydrogenic bonds. This contributes to the modification of the three‐dimensional structure of proteins hence the alteration of their function. These effects have encouraged research‐ ers to combine propolis with antibiotics in order to overcome the problem of bacterial resistance to drugs [134]. A synergy has been observed between EEP, vancomycin and oxacillin; antibiotics that inhibit the synthesis of the bacterial cell wall, against S. pyogenes, VRE ATCC 51299 and MRSA NCTC 10442 [135]. Also, synergism was present between EEP and drugs targeting microbial ribosomes (neomycin) but absent with drugs interfer‐ ing in the biosynthesis of folic acid or DNA (ciprofloxacin and norfloxacin) as well as those inhibiting metabolic pathways (cotrimoxazole) [134]. EEP has proved to be the most ef‐ fective when combined with antibiotics that interfere with bacterial protein biosynthesis such as tetracycline, linezolid, chloramphenicol, gentamicin, tobramycin and netilmicin against MRSA and MSSA [127].
Propolis is widely used in medicine due to its various bioactivities. The effect of top‐ ical propolis extract was tested on facial vulgaris acne where subjects were treated with a topical solution of ethanolic extract of propolis. This solution showed a significant bacte‐ riological activity on Propionibacterium acnes and Staphylococcus epidermidis, as well as be‐ ing effective against facial acne [136]. To test the efficiency of propolis in inhibiting dental caries, propolis fluoride was applied to children’s teeth with active dentinal carries sur‐ face. Carries were stopped in 99.80% of the cases after just one month of application with‐ out causing any black discoloration of the teeth [137]. In another study, researchers treated patients suffering from chronic periodontitis with an irrigation of the selected sites with a hydro alcoholic solution of propolis extract propolis. Results showed that propolis exhib‐ its a considerable activity against chronic periodontitis. This treatment was more effective than the conventional treatment as it significantly decreased the viability of P. gingivalis [138].
6. Conclusions
References
- D’Apolito Pessoa, S.M.; Balestieri, F.C.; de LM Balestieri, J.B.P. Pollen harvest by Apis mellifera L. (Hymenoptera: Apidae) in the Dourados region, Mato Grosso do Sul state (Brazil). Acta Bot. Bras. 2010, 24, 898–904.
- Greenleaf, S.S.; Kremen, C. Wild bees enhance honey bees’ pollination of hybrid sunflower. Proc. Natl. Acad. Sci. USA 2006, 103, 13890–13895.
- Bogdanov, S. Pollen: Nutrition, Functional Properties, Health. Bee Prod. Sci. 2016, 20, 350.
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical aspects of propolis research in modern times. Evid Based Complement. Altern. Med. 2013, 2013, 964149.
- Kassim, M.; Achoui, M.; Mansor, M.; Yusoff, K.M. The inhibitory effects of Gelam honey and its extracts on nitric oxide and prostaglandin E2 in inflammatory tissues. Fitoterapia 2010, 81, 1196–1201.
- Khalil, W.K.B.; Assaf, N.; ElShebiney, S.A.; Salem, N.A. Neuroprotective effects of bee venom acupuncture therapy against rotenone-induced oxidative stress and apoptosis. Neurochem. Int. 2015, 80, 79–86.
- Arteaga, V.; Lamas, A.; Regal, P.; Vazquez, B.; Miranda, J.; Cepeda, A.; Franco, C.M. Antimicrobial activity of apitoxin from Apis mellifera in Salmonella enterica strains isolated from poultry and its effects on motility, biofilm formation and gene expression. Microb. Pathog. 2019, 137, 103771.
- Abd El-Wahed, A.A.; Khalifa, S.A.M.; Sheikh, B.Y.; Farag, M.A.; Saeed, A.; Larik, F.A. Chapter 13 Bee Venom Composition: From Chemistry to Biological Activity. In Studies in Natural Products Chemistry; Rahman, A.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2019.
- Yaacoub, C.; Rifi, M.; El-Obeid, D.; Mawlawi, H.; Sabatier, J.-M.; Coutard, B.; Fajloun, Z. The Cytotoxic Effect of Apis mellifera Venom with a Synergistic Potential of Its Two Main Components-Melittin and PLA2-On Colon Cancer HCT116 Cell Lines. Molecules 2021, 26, 2264.
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front Pharmacol. 2017. Available online: (accessed on 2 June 2021).
- Paulino, N.; Abreu, S.R.L.; Uto, Y.; Koyama, D.; Nagasawa, H.; Hori, H.; Koca-Caliskan, U.; AlAjmi, M.F.; Hassan, M.; Wahabi, H.A.; et al. Anti-inflammatory effects of a bioavailable compound, Artepillin C, in Brazilian propolis. Eur. J. Pharmacol. 2008, 587, 296–301.
- Schnitzler, P.; Neuner, A.; Nolkemper, S.; Zundel, C.; Nowack, H.; Sensch, K.H.; Reichling, J. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother. Res. 2010, 1, S20–S28.
- Buchwald, R.; Breed, M.D.; Greenberg, A.R.; Otis, G. Interspecific variation in beeswax as a biological construction material. J. Exp. Biol. 2006, 209, 3984–3989.
- Bridi, R.; Atala, E.; Pizarro, P.N.; Montenegro, G. Honeybee Pollen Load: Phenolic Composition and Antimicrobial Activity and Antioxidant Capacity. J. Nat. Prod. 2019, 82, 559–565.
- Campos, M.; Frigerio, C.; Lopes, J.; Bogdanov, S. What is the future of Bee-Pollen? J. ApiProduct ApiMedical Sci. 2010, I, 131–144.
- Ahmed, R.; Tanvir, E.M.; Hossen, M.S.; Afroz, R.; Ahmmed, I.; Rumpa, N.-E.-N.; Paul, S.; Gan, S.H.; Sulaiman, S.A.; Khalil, M.I. Antioxidant Properties and Cardioprotective Mechanism of Malaysian Propolis in Rats. Evid. Based Complementary Altern. Med. 2017, 2017, e5370545.
- Inoue, S.; Koya-Miyata, S.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal Jelly prolongs the life span of C3H/HeJ mice: Correlation with reduced DNA damage. Exp. Gerontol. 2003, 38, 965–969.
- Simúth, J.; Bíliková, K.; Kovácová, E.; Kuzmová, Z.; Schroder, W. Immunochemical approach to detection of adulteration in honey: Physiologically active royal jelly protein stimulating TNF-alpha release is a regular component of honey. J. Agric. Food Chem. 2004, 52, 2154–2158.
- Nakaya, M.; Onda, H.; Sasaki, K.; Yukiyoshi, A.; Tachibana, H.; Yamada, K. Effect of Royal Jelly on Bisphenol A-Induced Proliferation of Human Breast Cancer Cells. Biosci. Biotechnol. Biochem. 2007, 71, 253–255.
- Kohno, K.; Okamoto, I.; Sano, O.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal jelly inhibits the production of proinflammatory cytokines by activated macrophages. Biosci. Biotechnol. Biochem. 2004, 68, 138–145.
- Hattori, N.; Ohta, S.; Sakamoto, T.; Mishima, S.; Furukawa, S. Royal jelly facilitates restoration of the cognitive ability in trimethyltin-intoxicated mice. Evid Based Complement Altern. Med. 2011, 2011, 165968.
- Blasa, M.; Candiracci, M.; Accorsi, A.; Piacentini, M.; Albertini, M.; Piatti, E. Raw Milefiori honey is packed full of antioxidant. Food Chem. 2006, 97, 217–222.
- Ahmed, A.K.J.; Hoekstra, M.J.; Hage, J.J.; Kariml, R.B. Honey-medicated dressing: Transformation of an ancient remedy into modern therapy. Ann. Plast. Surg. 2003, 50, 143–147.
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complementary Altern. Med. 2013, 13, 43.
- Khalil, M.; Moniruzzaman, M.; Boukraâ, L.; Benhanifia, M.; Islam, M.; Sulaiman, S.A.; Gan, S.H. Physicochemical and Antioxidant Properties of Algerian Honey. Molecules 2012, 17, 11199–11215.
- Brown, E.; O’Brien, M.; Georges, K.; Suepaul, S. Physical Characteristics and Antimicrobial Properties of Apis Mellifera, Frieseomelitta Nigra and Melipona Favosa Bee Honeys from Apiaries in Trinidad and Tobago. BMC Complement Med. Ther. 2020, 20. Available online: (accessed on 27 April 2021).
- Gheldof, N.; Wang, X.-H.; Engeseth, N.J. Identification and quantification of antioxidant components of honeys from various floral sources. J. Agric. Food Chem. 2002, 50, 5870–5877.
- Da CAzeredo, L.; Azeredo, M.A.; De Souza, S.R.; Dutra, V.M. Protein contents and physicochemical properties in honey samples of Apis mellifera of different floral origins. Food Chem. 2003, 80, 249–254.
- Kumar, P.; Sindhu, R.K.; Narayan, S.; Singh, I. Honey collected from different floras of Chandigarh Tricity: A comparative study involving physicochemical parameters and biochemical activities. J. Diet Suppl. 2010, 7, 303–313.
- Kuś, P.M.; Szweda, P.; Jerković, I.; Tuberoso, C.I.G. Activity of Polish unifloral honeys against pathogenic bacteria and its correlation with colour, phenolic content, antioxidant capacity and other parameters. Lett. Appl. Microbiol. 2016, 62, 269–276.
- Bang, L.M.; Buntting, C.; Molan, P. The effect of dilution on the rate of hydrogen peroxide production in honey and its implications for wound healing. J. Altern. Complement Med. 2003, 9, 267–273.
- Sowa, P.; Grabek-Lejko, D.; Wesołowska, M.; Swacha, S.; Dżugan, M. Hydrogen peroxide-dependent antibacterial action of Melilotus albus honey. Lett. Appl. Microbiol. 2017, 65, 82–89.
- Taormina, P.J.; Niemira, B.A.; Beuchat, L.R. Inhibitory activity of honey against foodborne pathogens as influenced by the presence of hydrogen peroxide and level of antioxidant power. Int. J. Food Microbiol. 2001, 69, 217–225.
- Brudzynski, K.; Abubaker, K.; Miotto, D. Unraveling a mechanism of honey antibacterial action: Polyphenol/H₂O₂-induced oxidative effect on bacterial cell growth and on DNA degradation. Food Chem. 2012, 133, 329–336.
- Abdulrhman, M.A.; Mekawy, M.A.; Awadalla, M.M.; Mohamed, A.H. Bee honey added to the oral rehydration solution in treatment of gastroenteritis in infants and children. J. Med. Food 2010, 13, 605–609.
- Ratiu, I.A.; Al-Suod, H.; Bukowska, M.; Ligor, M.; Buszewski, B. Correlation Study of Honey Regarding their Physicochemical Properties and Sugars and Cyclitols Content. Molecules 2019, 20, 34.
- Kwakman, P.H.S.; te Velde, A.A.; de Boer, L.; Speijer, D. Vandenbroucke-Grauls CMJE, Zaat SAJ. How honey kills bacteria. FASEB J. 2010, 24, 2576–2582.
- Adams, C.J.; Manley-Harris, M.; Molan, P.C. The origin of methylglyoxal in New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 2009, 344, 1050–1053.
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489.
- Kato, Y.; Umeda, N.; Maeda, A.; Matsumoto, D.; Kitamoto, N.; Kikuzaki, H. Identification of a Novel Glycoside, Leptosin, as a Chemical Marker of Manuka Honey. J. Agric. Food Chem. 2012, 60, 3418–3423.
- Jenkins, R.; Cooper, R. Improving antibiotic activity against wound pathogens with manuka honey in vitro. PLoS ONE 2012, 7, e45600.
- Müller, P.; Alber, D.G.; Turnbull, L.; Schlothauer, R.C.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Synergism between Medihoney and Rifampicin against Methicillin-Resistant Staphylococcus aureus (MRSA). PLoS ONE 2013, 8, e57679.
- Gethin, G.; Cowman, S.; Conroy, R. The impact of Manuka honey dressings on the surface pH of chronic wounds. Int. Wound J. 2008, 5, 185–194.
- Brudzynski, K.; Sjaarda, C. Antibacterial compounds of Canadian honeys target bacterial cell wall inducing phenotype changes, growth inhibition and cell lysis that resemble action of β-lactam antibiotics. PLoS ONE 2014, 9, e106967.
- Mokaya, H.O.; Bargul, J.L.; Irungu, J.W.; Lattorff, H.M.G. Bioactive constituents, in vitro radical scavenging and antibacterial activities of selected Apis mellifera honey from Kenya. Int. J. Food Sci. Technol. 2020, 55, 1246–1254.
- Cooper, R.A.; Molan, P.C.; Harding, K.G. The sensitivity to honey of Gram-positive cocci of clinical significance isolated from wounds. J. Appl. Microbiol. 2002, 93, 857–863.
- Brudzynski, K.; Lannigan, R. Mechanism of Honey Bacteriostatic Action Against MRSA and VRE Involves Hydroxyl Radicals Generated from Honey’s Hydrogen Peroxide. Front. Microbiol. 2012, 3, 36.
- Header, E.; Hashish, A.; ElSawy, N.; Alkushi, A.; El-Boshy, M.; Elsawy, N. Gastroprotective effect of dietary honey against acetylsalicylate induced expermental ulcer in albino rat. Life Sci. J. 2016, 13, 42–47.
- Brown, H.; Metters, G.; Hitchings, M.; Wilkinson, T.; Sousa, L.; Cooper, J.; Dance, H.; Atterbury, R.; Jenkins, R. Antibacterial and anti-virulence activity of manuka honey against genetically diverse Staphylococcus pseudintermedius. Appl. Environ. Microbiol. 2020, 86, e01768-20.
- Alnaqdy, A.; Al-Jabri, A.; Al Mahrooqi, Z.; Nzeako, B.; Nsanze, H. Inhibition effect of honey on the adherence of Salmonella to intestinal epithelial cells in vitro. Int. J. Food Microbiol. 2005, 103, 347–351.
- Badet, C.; Quero, F. The in vitro effect of manuka honeys on growth and adherence of oral bacteria. Anaerobe 2011, 17, 19–22.
- Alandejani, T.; Marsan, J.; Ferris, W.; Slinger, R.; Chan, F. Effectiveness of honey on Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Otolaryngol. Head Neck Surg. 2009, 141, 114–118.
- Cooper, R.A.; Wigley, P.; Burton, N.F. Susceptibility of multiresistant strains of Burkholderia cepacia to honey. Lett. Appl. Microbiol. 2000, 31, 20–24.
- Wilkinson, J.M.; Cavanagh, H.M.A. Antibacterial activity of 13 honeys against Escherichia coli and Pseudomonas aeruginosa. J. Med. Food. 2005, 8, 100–103.
- Moussa, A.; Noureddine, D.; Mohamed, H.S.; Abdelmelek, M.; Saad, A. Antibacterial activity of various honey types of Algeria against Staphylococcus aureus and Streptococcus pyogenes. Asian Pac. J. Trop. Med. 2012, 5, 773–776.
- Lusby, P.E.; Coombes, A.L.; Wilkinson, J.M. Bactericidal activity of different honeys against pathogenic bacteria. Arch Med. Res. 2005, 36, 464–467.
- Eick, S.; Schäfer, G.; Kwieciński, J.; Atrott, J.; Henle, T.; Pfister, W. Honey a potential agent against Porphyromonas gingivalis: An in vitro study. BMC Oral Health 2014, 25, 24.
- Bucekova, M.; Bugarova, V.; Godocikova, J.; Majtan, J. Demanding New Honey Qualitative Standard Based on Antibacterial Activity. Foods 2020, 9, 1263.
- Combarros-Fuertes, P.M.; Estevinho, L.; Teixeira-Santos, R.G.; Rodrigues, A.; Pina-Vaz, C.; Fresno, J.M.; Tornadijo, M.E. Antibacterial Action Mechanisms of Honey: Physiological Effects of Avocado, Chestnut, and Polyfloral Honey upon Staphylococcus aureus and Escherichia coli. Molecules 2020, 25, 1252.
- Adeleke, O.E.; Olaitan, J.O.; Okpekpe, E.L. Comparative Antibacterial Activity of Honey and Gentamicin Against Escherichia Coli and Pseudomonas Aeruginosa. Ann. Burn. Fire Disasters 2006, 19, 201–204.
- Al-Jabri, A.A.; Al-Hosni, S.A.; Nzeako, B.C.; Al-Mahrooqi, Z.H.; Nsanze, H. Antibacterial activity of Omani honey alone and in combination with gentamicin. Saudi Med. J. 2005, 26, 767–771.
- Julianti, E.; Rajah, K.K.; Fidrianny, I. Antibacterial Activity of Ethanolic Extract of Cinnamon Bark, Honey, and Their Combination Effects against Acne-Causing Bacteria. Sci. Pharm. 2017, 85, 19.
- Campeau, M.E.M.; Patel, R. Antibiofilm Activity of Manuka Honey in Combination with Antibiotics. Int. J. Bacteriol. 2014, 2014, 795281.
- Liu, M.; Lu, J.; Müller, P.; Turnbull, L.; Burke, C.M.; Schlothauer, R.C.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Antibiotic-specific differences in the response of Staphylococcus aureus to treatment with antimicrobials combined with manuka honey. Front. Microbiol. 2014, 5, 779.
- Jenkins, R.; Cooper, R. Synergy between oxacillin and manuka honey Sensitizes methicillin-resistant Staphylococcus aureus to oxacillin. J. Antimicrob. Chemother. 2012, 67, 1405–1407.
- Blaser, G.; Santos, K.; Bode, U.; Vetter, H.; Simon, A. Effect of medical honey on wounds colonised or infected with MRSA. J. Wound Care 2007, 16, 325–328.
- Boyanova, L.; Ilieva, J.; Gergova, G.; Vladimirov, B.; Nikolov, R.; Mitov, I. Honey and green/black tea consumption may reduce the risk of Helicobacter pylori infection. Diagn. Microbiol. Infect. Dis. 2015, 82, 85–86.
- Yousaf, I.; Ishaq, I.; Hussain, M.B.; Inaam, S.; Saleem, S.; Qamar, M.U. Antibacterial activity of Pakistani Beri honey compared with silver sulfadiazine on infected wounds: A clinical trial. J. Wound Care 2019, 28, 291–296.
- Szweda, P. Antimicrobial Activity of Honey [Internet]. Honey Analysis. IntechOpen. 2017. Available online: (accessed on 5 June 2021).
- Cooper, R.A.; Jenkins, L.; Henriques, A.F.M.; Duggan, R.S.; Burton, N.F. Absence of bacterial resistance to medical-grade manuka honey. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1237–1241.
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria? Antibiotics 2020, 9, 774.
- Blair, S.E.; Cokcetin, N.N.; Harry, E.J.; Carter, D.A. The unusual antibacterial activity of medical-grade Leptospermum honey: Antibacterial spectrum, resistance and transcriptome analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1199–1208.
- Hermanns, R.; Mateescu, C.; Thrasyvoulou, A.; Tananaki, C.; Wagener, F.; Cremers, N. Defining the standards for medical grade honey. J. Apic. Res. 2019, 59, 1–11.
- Molan, P.C.; Allen, K.L. The Effect of Gamma-irradiation on the Antibacterial Activity of Honey. J. Pharm. Pharmacol. 1996, 48, 1206–1209.
