Propofol is a commonly used intravenous sedative for ventilator-dependent patients. Its advantage over other sedative agents, such as benzodiazepines, is in its rapid onset and short half-life which allows for daily awakening and spontaneous breathing trials. The use of propofol is recommended by the Brain Trauma Foundation for the treatment of elevated intracranial pressure and sometimes prolonged continuous large doses are required.
- propofol
- enteral nutrition
- parenteral nutrition
- fat emulsions
- critical illness
- nutritional requirements
- hypnotics and sedatives
- food–drug interactions
1. Introduction
The use of propofol is recommended by the Brain Trauma Foundation for the treatment of elevated intracranial pressure [1] and sometimes prolonged continuous large doses are required. Prolonged use of large doses can be problematic in the nutritional management of these patients since propofol is formulated in a 10% lipid emulsion as either soybean oil in the United States or available in a mixed oil formulation internationally. As a result, complications associated with caloric overfeeding, hypertriglyceridemia, and inadequate protein intake may occur for patients receiving propofol therapy [2]. The intent of this entreviewy is to examine caloric intake associated with propofol therapy and to describe some strategies to avoid overfeeding that will still meet the increased protein needs of critically ill patients who require enteral nutrition (EN) or parenteral nutrition (PN) therapy.
2. Complications Associated with Hypertriglyceridemia and Caloric Overfeeding
3. The Rationale and Dilemma for Providing Sufficient Protein Intake
4. Strategies to Avoid Overfeeding with Calories and to Maintain or Improve Protein Intake
4.1. Parenteral Nutrition
4.2. Enteral Nutrition
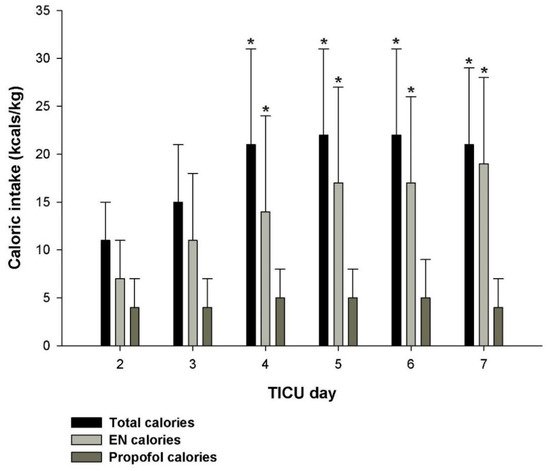
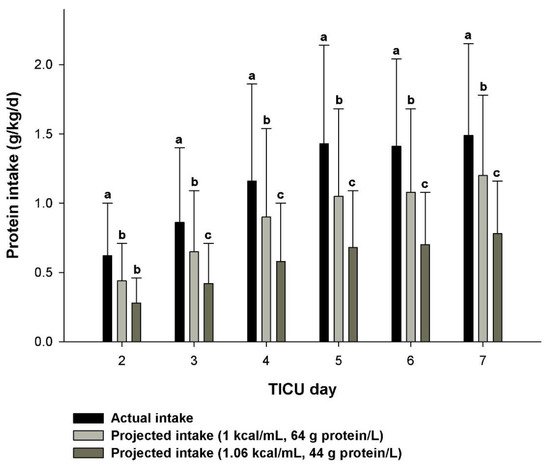
References
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the management of severe traumatic brain injury. Neurosurgery 2017, 80, 6–15.
- Buckley, C.T.; Dickerson, R.N. Propofol: A risk factor for caloric overfeeding and inadequate protein delivery. Hosp. Pharm. 2020, 55, 151–152.
- Llop, J.; Sabin, P.; Garau, M.; Burgos, R.; Perez, M.; Masso, J.; Cardona, D.; Sanchez Segura, J.M.; Garriga, R.; Redondo, S.; et al. The importance of clinical factors in parenteral nutrition-associated hypertriglyceridemia. Clin. Nutr. 2003, 22, 577–583.
- Miles, J.M.; Park, Y.; Harris, W.S. Lipoprotein liapse and triglyceride-rich lipoprotein metabolism. Nutr. Clin. Pract. 2001, 16, 273–279.
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter Enter. Nutr. 2016, 40, 159–211.
- Seidner, D.L.; Mascioli, E.A.; Istfan, N.W.; Porter, K.A.; Selleck, K.; Blackburn, G.L.; Bistrian, B.R. Effects of long-chain triglyceride emulsions on reticuloendothelial system function in humans. JPEN J. Parenter Enter. Nutr. 1989, 13, 614–619.
- Buckley, C.T.; Van Matre, E.T.; Fischer, P.E.; Minard, G.; Dickerson, R.N. Improvement in protein delivery for critically ill patients requiring high-dose propofol therapy and enteral nutrition. Nutr. Clin. Pract. 2021, 36, 212–218.
- Ibarra-Pastrana, E.; Serralde-Zuniga, A.; Calderon de la Barca, A.M. Critical energy and protein deficits with high contribution of non-nutrient calories after one week into an intensive care unit. Clin. Nutr. 2020, 40, 632.
- Taylor, S.J.; Bowles, J.; Jewkes, C. Propofol use precludes prescription of estimated nitrogen requirements. J. Intensive Care Med. 2005, 20, 111–117.
- Talpers, S.S.; Romberger, D.J.; Bunce, S.B.; Pingleton, S.K. Nutritionally associated increased carbon dioxide production. Excess total calories vs high proportion of carbohydrate calories. Chest 1992, 102, 551–555.
- Rosmarin, D.K.; Wardlaw, G.M.; Mirtallo, J. Hyperglycemia associated with high, continuous infusion rates of total parenteral nutrition dextrose. Nutr. Clin. Pract. 1996, 11, 151–156.
- Zusman, O.; Theilla, M.; Cohen, J.; Kagan, I.; Bendavid, I.; Singer, P. Resting energy expenditure, calorie and protein consumption in critically ill patients: A retrospective cohort study. Crit. Care 2016, 20, 367.
- Allingstrup, M.J.; Esmailzadeh, N.; Wilkens Knudsen, A.; Espersen, K.; Hartvig Jensen, T.; Wiis, J.; Perner, A.; Kondrup, J. Provision of protein and energy in relation to measured requirements in intensive care patients. Clin. Nutr. 2012, 31, 462–468.
- Yeh, D.D.; Fuentes, E.; Quraishi, S.A.; Lee, J.; Kaafarani, H.M.A.; Fagenholz, P.; Butler, K.; DeMoya, M.; Chang, Y.; Velmahos, G. Early protein inadequacy is associated with longer intensive care unit stay and fewer ventilator-free days: A retrospective analysis of patients with prolonged surgical intensive care unit stay. JPEN J. Parenter Enter. Nutr. 2018, 42, 212–218.
- Nicolo, M.; Heyland, D.K.; Chittams, J.; Sammarco, T.; Compher, C. Clinical outcomes related to protein delivery in a critically ill population: A multicenter, multinational observation study. JPEN J. Parenter Enter. Nutr. 2016, 40, 45–51.
- Berger, M.M.; Soguel, L.; Charriere, M.; Theriault, B.; Pralong, F.; Schaller, M.D. Impact of the reduction of the recommended energy target in the ICU on protein delivery and clinical outcomes. Clin. Nutr. 2017, 36, 281–287.
- Choban, P.; Dickerson, R.; Malone, A.; Worthington, P.; Compher, C. American Society for, Parenteral and Enteral Nutrition, ASPEN Clinical guidelines: Nutrition support of hospitalized adult patients with obesity. JPEN J. Parenter Enter. Nutr. 2013, 37, 714–744.
- Choban, P.S.; Dickerson, R.N. Morbid obesity and nutrition support: Is bigger different? Nutr. Clin. Pract. 2005, 20, 480–487.
- Dickerson, R.N.; Pitts, S.L.; Maish, G.O., 3rd; Schroeppel, T.J.; Magnotti, L.J.; Croce, M.A.; Minard, G.; Brown, R.O. A reappraisal of nitrogen requirements for patients with critical illness and trauma. J. Trauma. Acute Care Surg. 2012, 73, 549–557.
- Scheinkestel, C.D.; Kar, L.; Marshall, K.; Bailey, M.; Davies, A.; Nyulasi, I.; Tuxen, D.V. Prospective randomized trial to assess caloric and protein needs of critically Ill, anuric, ventilated patients requiring continuous renal replacement therapy. Nutrition 2003, 19, 909–916.
- Dickerson, R.N.; Medling, T.L.; Smith, A.C.; Maish, G.O., 3rd; Croce, M.A.; Minard, G.; Brown, R.O. Hypocaloric, high-protein nutrition therapy in older vs younger critically ill patients with obesity. JPEN J. Parenter Enter. Nutr. 2013, 37, 342–351.
- Dickerson, R.N.; Maish, G.O., 3rd; Croce, M.A.; Minard, G.; Brown, R.O. Influence of aging on nitrogen accretion during critical illness. JPEN J. Parenter Enter. Nutr. 2015, 39, 282–290.
- Ferrie, S.; Herkes, R.; Forrest, P. Nutrition support during extracorporeal membrane oxygenation (ECMO) in adults: A retrospective audit of 86 patients. Intensive Care Med. 2013, 39, 1989–1994.
- Minnelli, N.; Gibbs, L.; Larrivee, J.; Sahu, K.K. Challenges of maintaining optimal nutrition dtatus in COVID-19 patients in intensive care settings. JPEN J. Parenter Enter. Nutr. 2020, 44, 1439–1446.
- Bousie, E.; van Blokland, D.; Lammers, H.J.; van Zanten, A.R. Relevance of non-nutritional calories in mechanically ventilated critically ill patients. Eur. J. Clin. Nutr. 2016, 70, 1443–1450.
- DeChicco, R.; Materese, L.; Hummell, A.C.; Speerhas, R.; Seidner, D.; Steiger, E. Contribution of calories from propofol to total energy intake. J. Am. Diet. Assoc. 1995, 95, A25.
- Morgan, L.M.; Dickerson, R.N.; Alexander, K.H.; Brown, R.O.; Minard, G. Factors causing interrupted delivery of enteral nutrition in trauma intensive care unit patients. Nutr. Clin. Pract. 2004, 19, 511–517.
- Dickerson, R.N.; Mitchell, J.N.; Morgan, L.M.; Maish, G.O., 3rd; Croce, M.A.; Minard, G.; Brown, R.O. Disparate response to metoclopramide therapy for gastric feeding intolerance in trauma patients with and without traumatic brain injury. JPEN J. Parenter Enter. Nutr. 2009, 33, 646–655.
- Sessler, C.N.; Gosnell, M.S.; Grap, M.J.; Brophy, G.M.; O’Neal, P.V.; Keane, K.A.; Tesoro, E.P.; Elswick, R.K. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care unit patients. Am. J. Respir. Crit. Care Med. 2002, 166, 1338–1344.
- Burke, J.F.; Wolfe, R.R.; Mullany, C.J.; Mathews, D.E.; Bier, D.M. Glucose requirements following burn injury. Parameters of optimal glucose infusion and possible hepatic and respiratory abnormalities following excessive glucose intake. Ann. Surg. 1979, 190, 274–285.
- Elwyn, D.H. Nutritional requirements of adult surgical patients. Crit. Care Med. 1980, 8, 9–20.
- Dickerson, R.N.; Rosato, E.F.; Mullen, J.L. Net protein anabolism with hypocaloric parenteral nutrition in obese stressed patients. Am. J. Clin. Nutr. 1986, 44, 747–755.
- Wilmore, D.W.; Aulick, L.H.; Mason, A.D.; Pruitt, B.A., Jr. Influence of the burn wound on local and systemic responses to injury. Ann. Surg. 1977, 186, 444–458.
- Hastings, J.; Ridley, E.J.; Bianchet, O.; Roodenburg, O.; Levkovich, B.; Scheinkestel, C.; Pilcher, D.; Udy, A. Does propofol sedation contribute to overall energy provision in mechanically ventilated critically ill adults? A retrospective observational study. JPEN J. Parenter Enter. Nutr. 2018, 42, 748–757.
- Castro, M.; Nogueira, P.B.; Ribeiro, F.A.; Bottairi, D.S.; Piovacari, S.F.; Assis, T.; Laselva, C.R.; Toledo, D.O. Relevance of non-nutritional calories by propofol in Covid-19 critically ill patients. Clin. Nutr. 2020, 40, 509.
- Richardson, M.; Saunders, J.; Quinn, C.; Halloran, T.O.; Riain, S.O. Improvements in goal protein achieved following a change from propofol 1% to propofol 2% and a change in enteral feeding protocol in the ICU. Clin. Nutr. 2018, 37, S55.
- Terblanche, E.; Remmington, C. Observational study evaluating the nutritional impact of changing from 1% to 2% propofol in a cardiothoracic adult critical care unit. J. Hum. Nutr. Diet. 2020, 34, 413–419.
- Kudsk, K.A.; Minard, G.; Croce, M.A.; Brown, R.O.; Lowrey, T.S.; Pritchard, F.E.; Dickerson, R.N.; Fabian, T.C. A randomized trial of isonitrogenous enteral diets after severe trauma. An immune-enhancing diet reduces septic complications. Ann. Surg. 1996, 224, 531–540, discussion 540-533.
- Dickerson, R.N.; Harris, S.L. Difficulty in administration of liquid protein solution via an enteral feeding tube. Am. J. Health Syst. Pharm. 2009, 66, 796–797.
- Dickerson, R.N.; Boschert, K.J.; Kudsk, K.A.; Brown, R.O. Hypocaloric enteral tube feeding in critically ill obese patients. Nutrition 2002, 18, 241–246.
