Tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) are two cytokines involved in the perpetuation of the chronic inflammation state characterizing rheumatoid arthritis (RA). Significant advances in the treatment of this pathology have been made over the past ten years, partially through the development of anti-TNF and anti-IL-1 therapies. However, major side effects still persist and new alternative therapies should be considered.
- rheumatoid arthritis
- TNF-α
- IL-1β
- ultra-low doses
- micro-immunotherapy
- anti-inflammatory medicines
- hormesis
- chronic inflammation
- inflammatory cytokines
1. An Introduction to Rheumatoid Arthritis and Micro-Immunotherapy
Rheumatoid arthritis (RA) is a widespread systemic autoimmune disease characterized by chronic inflammation of the articular membrane, synovial hyperplasia, and progressive degradation of cartilage and bone, leading to joint destruction. Patients with RA usually experience joint pain, swelling, tenderness, and stiffness, especially in the morning, and they also have to cope with fatigue and depression as the disease represents high personal and social burden[1]. Studies conducted in Northern European and North American areas evaluated RA prevalence at about 0.5–1%[2], whereas it appeared less frequently in low- and middle-income countries, where it is gauged at 0.4% and 0.37% in Southeast Asian and in Eastern Mediterranean regions, respectively[3]. This debilitating disease affects women at a rate double that in men, and in more aggressive forms[4].
As research progresses in delineating the drivers of this pathology, it appears that multiple immune factors, all integrated in a complex network and in a particular temporal frame, are involved in the onset and the progression of the disease. For instance, it is now well-established that numerous pro-inflammatory cytokines and growth factors perpetuate the chronic inflammation state of RA, in particular, interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α). Cytokines are typically secreted by immune cells in order to orchestrate diverse cellular functions, such as cell differentiation, activation, migration, survival or proliferation in a paracrine or autocrine manner. As they display pleiotropic effects, can either act synergistically or share redundancy of actions one with the others, a fine-tuned regulation of their interplay is of paramount interest in the proper immune system maintenance and the body’s homeostasis.
Significant advances in the treatment of this pathology have been made over the past ten years and the clinical arsenal mainly encompasses the so-called disease-modifying anti-rheumatic drugs (DMARDs) including methotrexate, sulfasalazine, hydroxychloroquine and leflunomide, the janus-activated kinase 3 (JAK3) inhibitor tofacitinib, glucocorticoids and biologic agents such as infliximab, etanercept, adalimumab, golimumab, and certolizumab pegol. All of them are used in both early and established RA (< or > 6 months), depending on patients’ values, preferences and comorbidities[5][6]. Regarding the pivotal role played by the resident/immune cells hosted in the synovial tissue in the pathogenesis of the disease, cell-targeted therapies have also been developed. Unfortunately, most of the therapeutics can only alleviate symptoms without intrinsically treating the root of the problem, ultimately leading to an incomplete remission.
In line with those prerequisites, novel treatments—sequentially able to modulate the expression of multiple immune players over time, as the stage of the pathology evolves—would be of great interest. 2LARTH® has been developed to attenuate the symptoms of rheumatic diseases including RA, within a holistic immunomodulatory approach. This strategy combines the use of several immune system mediators such as cytokines, growth factors, hormones, nucleic acids and specific nucleic acids (SNA®), in order to orchestrate both the innate and the adaptive/acquired immune responses. Low doses (LD) range from 1 CH to 7 CH and are used in MI to boost the immune system, activate or increase protein expression, while ultra-low doses (ULD) aim at modulating (from 8 to 12 CH) and lowering (higher than 12 CH) the expression of protein(s) with upregulated levels.
The first part of this review aims at delineating the pivotal roles played by IL-1β and TNF-α in RA physiopathology, leading to the development of anti-TNF and anti-IL-1 therapeutic agents. In a second part, an emphasis will be made on explaining the rationale of using multiple therapeutic targets, including both IL-1β and TNF-α in 2LARTH® medicine. Particular attention will be paid to the ULD of those two main pro-inflammatory factors in order to counteract their overexpression, through the lens of their molecular implication in RA pathogenesis.
2. RA and Cytokines: A TNF-α and IL-1β Crosstalk Modeled Both In Vitro and In Vivo
The synovial niche's crosstalk in RA conditions was studied by Saeki et al., in their in vitro murine arthritis tissue-derived cells model[7]. They recently published that synovial macrophages cultured within conditioned-medium from synovial fibroblasts display changes in genic expression characterized by an upregulation in the expression of inflammatory markers such as Nos2, TNF-α, IL-1β and CD86. Moreover, TNF-α secretion was also found to be induced by DNA stimulation in the RA context, as CpG-rich DNA from blood plasma isolated from an RA patient was shown to stimulate the TLR9-MyD88-NF-kB signaling pathway when added to the culture medium of PBMCs (peripheral blood mononuclear cells) from healthy donors, resulting in a significant increase in the IL-6 and TNF-α secretion[8]. Moreover, the TNF-α secretion induced in monocytes stimulated by LPS (lipopolysaccaride) + IFN-γ (interferon-γ) was significantly decreased when the cells were pretreated for one hour with the anti-TNF-α etanercept at either 2 or 8 µg/mL, whereas the anti-IL-1β anakinra (from 2.5 to 20 µg/mL) didn’t show any effect on the TNF-α secretion[9]. These data even strengthen the fact that TNF-α acts as a key player in the chronicity of the RA disease and its related inflammatory-vicious cycle, as its expression and secretion are induced by a plethora of factors on a broad number of cells from the synovial niche.
The increase of pro-inflammatory cytokines expression such as TNF-α, IL-1β, IL-6 and IL-17, inflammatory mediators like COX-2 and 5-LOX (5-lipoxygenase), as well as the reduction of anti-inflammatory markers such as IL-4 and IL-10 are the hallmark of synovial inflammation and cartilage damages and are extensively monitored parameters in in vivo RA models[10][11]. The study reporting the benefits of using intra-articular injections of the anti-TNF infliximab, loaded onto thermosensitive hydrogel to decrease pro-inflammatory cytokines within the synovial fluids of a rabbit model of RA induced by ovalbumin and complete Freund’s adjuvant, is another example illustrating the interplay between TNF-α and IL-1β in vivo[12]. Indeed, two and six weeks after injections, the intra-articular levels of both TNF-α and IL-1β were significantly decreased, compared with the saline-treated control.
3. Anti-IL-1β and -TNF-α Therapies: Effects and Side Effects of the Conventional Allopathic Doses
IL-1β antagonism has been performed in the context of RA through several options; either targeting the cytokine itself or its receptor. In patients undergoing active RA despite being treated with methotrexate, the addition of subcutaneous injections of 150 mg canakinumab every four weeks over a 12-weeks period improved their therapeutic response regarding the global assessment of disease-related parameters[13]. Kinetic studies and modeling simulations used to extract the dose-response relationships confirmed that 150 mg every four weeks allowed for the capture of the majority of IL-1β, lowering it below an EC50 allowing to improve the ACR (American College of Rheumatology) scores in patients with RA[14]. Moreover, canakinumab has been considered as a good option for young patients with severe and/or refractory forms of IL-1-driven RA, in order to avoid joint deformation[15].
Anakinra is a human IL-1R antagonist currently approved for the treatment of RA, leading to significant improvements in disease symptoms and quality of life, regarding pain, Larsen radiographic scores and erythrocytes sedimentation rates, slowing down both radiologic manifestations of joint damages and bone erosion[16][17]. Furthermore, the treatment with this agent was shown to have beneficial effects on inflammatory and metabolic parameters, allowing to discontinue the concomitant use of glucocorticoids and anti-diabetic drugs in patients displaying both RA and type 2 diabetes[18]. Anakinra is mainly used as a second-line treatment in patients previously treated with a failing anti-TNF-α therapy or experiencing cancer or infectious disease such as Mycobacterium tuberculosis infections[19].
In RA, anti-TNF treatment was shown to reduce the production of pro-inflammatory cytokines, including IL-1 and IL-6, thus contributing to the disturbance of the crosstalk between those pathology drivers[20]. Certolizumab pegol is the only PEGylated TNF inhibitor, where the PEG (polyethylene glycol) attachment allows to increase its half-life in vivo, while it may also contribute to a better pharmacologic distribution into the inflamed arthritic tissues, compared with non-PEGylated antibodies[21]. Other side effects of adalimumab include infections of the skin and the urogenital tract; hematologic affections, like leukopenia and anemia; metabolism and nutrition defects, like lipids and hepatic enzymes increased levels; nervous system infections, like cephalea; gastrointestinal related disorders, including abdominal pain and nausea; musculo-skeletal pain, and injection-site reactions, like erythema. A Brazilian study also reported another TNF-α blocker adverse effect, as the use of such therapeutic agents was shown to be associated with high risk of active mycobacterial infections in patients with chronic inflammatory arthritis including RA even if they did not present any evidence of latent tuberculosis infection[22].
In addition, the TNF-α signalization blockade has also been done by inhibiting the TNF-α binding to its receptor through etanercept, a fully human soluble TNF receptor Fc fusion protein, where a dimer of the extracellular domains of human TNFR2 is fused to the Fc portion of human IgG1. A phase IV study recently assessed the real-world safety and effectiveness of biosimilar etanercept in patients with RA, ankylosing spondylitis or psoriatic arthritis and receiving biosimilar etanercept injections, either 25 mg twice weekly or 50 mg once weekly[23]. The results reported that even if the HAQ (Health Assessment Questionnaire) scores decreased from 1.32 ± 0.77 at baseline to 0.81 ± 0.61 at 12 months in patients with RA and psoriatic arthritis (p < 0.01), some patients still reported adverse effects such as injection-site reactions, abdominal pain and upper respiratory tract infections. The European Medicines Agency reports that the more frequent side effects for this medicine are infections such as bronchitis, cystitis and cutaneous affections, as well as allergic reactions[24].
Interestingly, besides the above-mentioned unwanted side effects, a meta-analysis highlighted that TNF-α antagonists may have a beneficial effect on aortic stiffness, therefore related to cardiovascular risk, which RA patients are more prone to[25].
4. Micro-Immunotherapy: A Multiple Immune-Targeted Ultra-Low-Dose-Based Strategy to Calm Chronic Inflammation in RA
One of the main challenges of immunotherapy lies in the feasibility of the immune system management by a proper targeting of the immunopathological activity without further affecting the immunosurveillance. Thus, by combining both the advantages of using the immune system players themselves and extremely low dosages, MI could be at a crossroads in the RA treatment too, as the side effects of a conventional treatment would be reduced and/or avoided.
The 2LARTH® is a medicine that was developed to attenuate the symptoms of rheumatic diseases including RA and which is currently notified as a homeopathic medicine under notification number 1507CH36 F1 by the Federal Agency for Medicines and Health Products in Belgium. This medicine consists of different capsules, each one intended to be taken according to the order in which they are packaged in the blister (from 1 to 10), to give daily information to the body. All the capsules are composed of the same cytokines/factors at various ULD depending on the capsule number.
In the same manner as for the evaluation of the anti-inflammatory effects of one single cytokine at a time, IL-1β or TNF-α at 27 CH, Floris et al. reported that 2LARTH® has a significant impact in reducing IL-1β secretion in human enriched monocytes stimulated with LPS[26]. Moreover, the secreted level of TNF-α was also reduced at concentrations ranging from 2.25 to 22 mM in the same conditions, as well as the IL-6 secretion, at 11 and 22 mM. Furthermore, the effects of the treatment have been evaluated in vivo, in a CIA mice model, in which a daily treatment respecting the sequential order of 2LARTH® was administered by oral gavage, starting 30 days after the first immunization[27]. Results showed its efficacy in reducing the clinical score, the degree of edema and the inflammation in treated animals, compared with the control ones.
The “three birds with one stone” strategy of 2LARTH® is illustrated in the Figure 1 below, as IL-1β, TNF-α, and IL-2 are the principal therapeutic targets, all three used at ULD. We have widely discussed the first two targets, and less dwelt about the third.
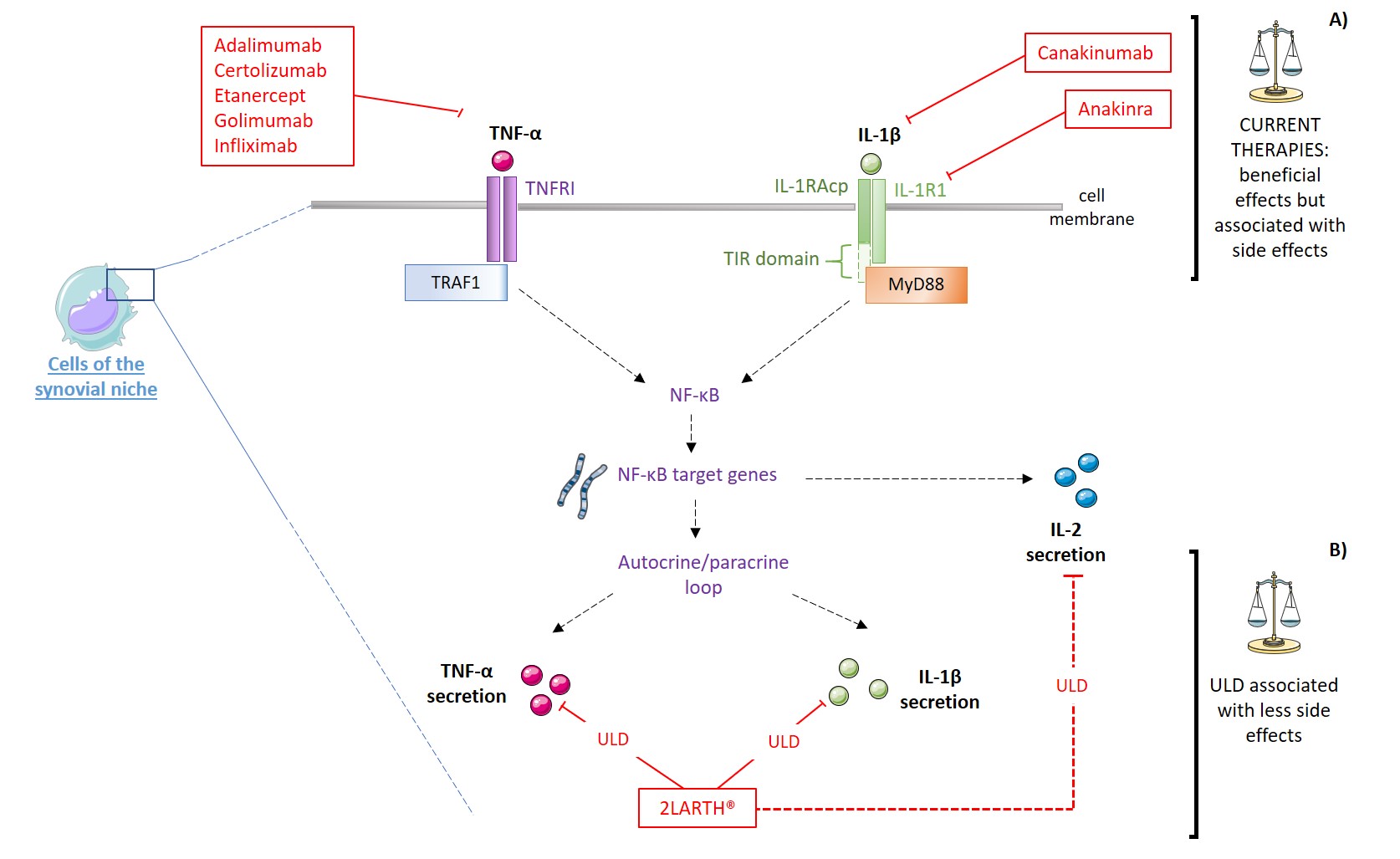
Figure 1: Schematic representation of the potential beneficial effects of ULD: IL-1R1: Interleukin-1 type-I receptor; IL-1RAcP: IL-1 receptor accessory protein; MAPK: Mitogen-activated protein kinase; MyD88: Myeloid and differentiation primary response 88; NF-κB: Nuclear factor-κB; TIR: Toll-IL-1 receptor; TNFR1: TNF receptor type 1; TRAF1: TNF receptor-associated factor 1; ULD: Ultra-Low dose. Red plain lines represent inhibition mechanisms, red dotted lines represent modulatory mechanisms.
Little is known about the genetic background of IL-2 in RA, but it was found that specific diplotypes, meaning the combination of haplotypes from both parents, in occurrence, the IL-2 TG: GG was positively associated with RA in the Turkish population[28]. Interestingly, the implication of this cytokine in joint-related pathologies seems to be dual and its use as a therapeutic target may be approached in a modulatory manner as contradictory data were addressed regarding this cytokine in this particular context. For instance, Paradowska-Gorycka et al. found that patients affected with RA or osteoarthritis have higher serum levels of IL-2 than the healthy controls[29], whereas Wang et al. reported an opposite trend, as the serum level of IL-2 was downregulated in RA patients group compared with healthy one[30].
A study focused on deciphering if the cytokine profile found in RA patients serum changed over the course of the disease showed that, 3 months after the initial diagnosis, IL-1β was positively correlated with two of the acute phase inflammatory reactants: CRP (C reactive protein) (R2= 0.642) and ESR (erythrocyte sedimentation rate) (R2= 0.579)[31]. This study conducted in Poland would need to be generalized to more patients, but it reinforces the fact that a holistic approach to RA treatment, with multiple cytokine targets, could be highly beneficial for patients. These results are in line with the fact that both IL-2 and TNF-α were found to be parts of a specific pro-inflammatory cytokine profile also defined by high levels of CXCL10 and IL-6, characterizing severe outcomes in systemic autoimmune diseases[32].
The wide therapeutic potential of IL-2 in many autoimmune and inflammatory diseases can be illustrated by the fact that, when the dose is high, IL-2 was reported to promote the proliferation of effector T cells, while, when the dose is low, it allowed an activation of the Tregs[33]. In addition, the effect of low dose IL-2 (5 IU/mL) was also shown to inhibit osteoclastogenesis in vitro, a mechanism which is implicated in the bone degradation also occurring in RA[34]. As the rationale of using IL-2 in the 2LARTH® is based on its immunomodulatory functions, the 10–12 CH dilution range employed in the sequence of the medicine allows the body’s responses to freely adjust by themselves, depending on the organism-specific needs at a particular time (Figure 1).
More preclinical studies are still needed to understand its mode of action, the synergy of its multiple regulators, the benefits of its sequential strategy, and clinical studies are indispensable to confirm and bring out the potential benefit of MI in the context of RA treatment.
References
- Frederick Wolfe; Kaleb Michaud; Predicting depression in rheumatoid arthritis: The signal importance of pain extent and fatigue, and comorbidity. Arthritis Care & Research 2009, 61, 667-673, 10.1002/art.24428.
- Yannis Alamanos; Alexandros A Drosos; Epidemiology of adult rheumatoid arthritis. Autoimmunity Reviews 2005, 4, 130-136, 10.1016/j.autrev.2004.09.002.
- Rudan Igor; Sidhu Simrita; Papana Angeliki; Meng Shi-Jiao; Xin-Wei Yu; Wang Wei; Campbell-Page Ruth M; Demaio Alessandro Rhyll; Nair Harish; Sridhar Devi; et al.Theodoratou EvropiDowman BenAdeloye DaviesMajeed AzeemCar JosipCampbell HarryChan Kit YeeNull Null Prevalence of rheumatoid arthritis in low– and middle–income countries: A systematic review and analysis. Journal of Global Health 2015, 5, 0, 10.7189/jogh.05.010409.
- Yannis Alamanos; Paraskevi V. Voulgari; Alexandros A. Drosos; Incidence and Prevalence of Rheumatoid Arthritis, Based on the 1987 American College of Rheumatology Criteria: A Systematic Review. Seminars in Arthritis and Rheumatism 2006, 36, 182-188, 10.1016/j.semarthrit.2006.08.006.
- Jasvinder A. Singh; Kenneth G. Saag; S. Louis Bridges Jr.; Elie A. Akl; Raveendhara Bannuru; Matthew Sullivan; Elizaveta Vaysbrot; Christine McNaughton; Mikala Osani; Robert H. Shmerling; et al.Jeffrey R. CurtisDaniel E. FurstDeborah ParksArthur KavanaughJames O'DellCharles KingAmye LeongEric L. MattesonJohn T. SchousboeBarbara DrevlowSeth GinsbergJames GroberE. William St.ClairElizabeth TindallAmy S. MillerTimothy McAlindon 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care & Research 2015, 68, 1-25, 10.1002/acr.22783.
- Adis Editorial; Tofacitinib. Drugs in R&D 2010, 10, 271-284, 10.2165/11588080-000000000-00000.
- Noritaka Saeki; Yuuki Imai; Reprogramming of synovial macrophage metabolism by synovial fibroblasts under inflammatory conditions. Cell Communication and Signaling 2020, 18, 1-14, 10.1186/s12964-020-00678-8.
- A.I. Speranskii; S.V. Kostyuk; E.A. Kalashnikova; N.N. Veiko; Speranskii A.I.; Kostyuk S.V.; Kalashnikova E.A.; Veiko N.N.; Enrichment of extracellular DNA from the cultivation medium of human peripheral blood mononuclears with genomic CpG rich fragments results in increased cell production of IL-6 and TNF-a via activation of the NF-kB signaling pathway. Biomeditsinskaya Khimiya 2016, 62, 331-340, 10.18097/pbmc20166203331.
- Hanna Schierbeck; Heidi Wähämaa; Ulf Andersson; Helena Erlandsson Harris; Immunomodulatory Drugs Regulate HMGB1 Release from Activated Human Monocytes. Molecular Medicine 2010, 16, 343-351, 10.2119/molmed.2010.00031.
- Rui Jing; Yanfei Ban; Weiheng Xu; Hua Nian; Yaoli Guo; Yiya Geng; Yuan Zang; Chengjian Zheng; Therapeutic effects of the total lignans from Vitex negundo seeds on collagen-induced arthritis in rats. Phytomedicine 2019, 58, 152825, 10.1016/j.phymed.2019.152825.
- Bing Lin; Hong Zhang; Xiang-Xiang Zhao; Khalid Rahman; Ying Wang; Xue-Qin Ma; Cheng-Jian Zheng; Qiao-Yan Zhang; Ting Han; Lu-Ping Qin; et al. Inhibitory effects of the root extract of Litsea cubeba (lour.) pers. on adjuvant arthritis in rats. Journal of Ethnopharmacology 2013, 147, 327-334, 10.1016/j.jep.2013.03.011.
- Weiying Chen; Zuhao Li; Zhenhong Wang; Hong Gao; Junyun Ding; Zhenzhou He; Intraarticular Injection of Infliximab-Loaded Thermosensitive Hydrogel Alleviates Pain and Protects Cartilage in Rheumatoid Arthritis. Journal of Pain Research 2020, ume 13, 3315-3329, 10.2147/jpr.s283518.
- Rieke Alten; Juan Gómez-Reino; Patrick Durez; Andre Beaulieu; Anthony Sebba; Gerhard Krammer; Ralph Preiss; Udayasankar Arulmani; Albert Widmer; Xavier Gitton; et al.Herbert Kellner Efficacy and safety of the human anti-IL-1beta monoclonal antibody canakinumab in rheumatoid arthritis: results of a 12-week, phase II, dose-finding study. BMC Musculoskeletal Disorders 2011, 12, 153-153, 10.1186/1471-2474-12-153.
- S Ait-Oudhia; Philip Lowe; D E Mager; Bridging Clinical Outcomes of Canakinumab Treatment in Patients With Rheumatoid Arthritis With a Population Model of IL-1β Kinetics. CPT: Pharmacometrics & Systems Pharmacology 2012, 1, 5-10, 10.1038/psp.2012.6.
- Nikolaos Marketos; Ilias Bournazos; Dimitrios Ioakimidis; Canakinumab for refractory RA: a case report.. Mediterranean Journal of Rheumatology 2018, 29, 170-172, 10.31138/mjr.29.3.170.
- Marty Mertens; Jasvinder A. Singh; Anakinra for Rheumatoid Arthritis: A Systematic Review. The Journal of Rheumatology 2009, 36, 1118-1125, 10.3899/jrheum.090074.
- Barry Bresnihan; Richard Newmark; Sean Robbins; Harry K Genant; Effects of anakinra monotherapy on joint damage in patients with rheumatoid arthritis. Extension of a 24-week randomized, placebo-controlled trial.. The Journal of Rheumatology 2004, 31, 0.
- Piero Ruscitti; Onorina Berardicurti; Paola Cipriani; Roberto Giacomelli; TRACK study group; Benefits of anakinra versus TNF inhibitors in rheumatoid arthritis and type 2 diabetes: long-term findings from participants furtherly followed-up in the TRACK study, a multicentre, open-label, randomised, controlled trial.. null 2021, 0, 0.
- Giulio Cavalli; Charles A. Dinarello; Treating rheumatological diseases and co-morbidities with interleukin-1 blocking therapies. Rheumatology 2015, 54, 2134-2144, 10.1093/rheumatology/kev269.
- P Charles; M J Elliott; D Davis; A Potter; J R Kalden; C Antoni; F C Breedveld; J S Smolen; G Eberl; K DeWoody; et al.M FeldmannR N Maini Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-alpha therapy in rheumatoid arthritis.. The Journal of Immunology 1999, 163, 0.
- Roger Palframan; Michael Airey; Adrian Moore; Alex Vugler; Andrew Nesbitt; Use of biofluorescence imaging to compare the distribution of certolizumab pegol, adalimumab, and infliximab in the inflamed paws of mice with collagen-induced arthritis. Journal of Immunological Methods 2009, 348, 36-41, 10.1016/j.jim.2009.06.009.
- Carina Mori Frade Gomes; Maria Teresa Terreri; Maria Isabel De Moraes-Pinto; Cássia Barbosa; Natália Pereira Machado; Maria Roberta Melo; Marcelo Medeiros Pinheiro; Incidence of active mycobacterial infections in Brazilian patients with chronic inflammatory arthritis and negative evaluation for latent tuberculosis infection at baseline - A longitudinal analysis after using TNFα blockers. Memórias do Instituto Oswaldo Cruz 2015, 110, 921-928, 10.1590/0074-02760150235.
- Farhad Gharibdoost; Amir-Hossein Salari; Mansour Salesi; Faegheh Ebrahimi Chaharom; Peyman Mottaghi; Mansour Hosseini; Maryam Sahebari; Mohammadali Nazarinia; Zahra Mirfeizi; Mohammadreza Shakibi; et al.Hamidreza MoussaviMansour KarimifarKarim MowlaHadi KarimzadehNassim AnjidaniAhmadreza Jamshidi Assessment of Treatment Safety and Quality of Life in Patients Receiving Etanercept Biosimilar for Autoimmune Arthritis (ASQA): A Multicenter Post-marketing Surveillance Study. Advances in Therapy 2021, 38, 1290-1300, 10.1007/s12325-020-01611-8.
- anx_146217_fr.pdf . ec.europa.eu. Retrieved 2021-7-6
- C. Vlachopoulos; A. Gravos; G. Georgiopoulos; Dimitrios Terentes-Printzios; N. Ioakeimidis; D. Vassilopoulos; K. Stamatelopoulos; D. Tousoulis; The effect of TNF-a antagonists on aortic stiffness and wave reflections: a meta-analysis. Clinical Rheumatology 2017, 37, 515-526, 10.1007/s10067-017-3657-y.
- Ilaria Floris; Kurt Appel; Thorsten Rose; Beatrice Lejeune; 2LARTH®, a micro-immunotherapy medicine, exerts anti-inflammatory effects in vitro and reduces TNF-α and IL-1β secretion. Journal of Inflammation Research 2018, ume 11, 397-405, 10.2147/jir.s174326.
- Ilaria Floris; Víctor García-González; Belen Palomares; Kurt Appel; Beatrice Lejeune; The Micro-Immunotherapy Medicine 2LARTH® Reduces Inflammation and Symptoms of Rheumatoid Arthritis In Vivo. International Journal of Rheumatology 2020, 2020, 1-9, 10.1155/2020/1594573.
- Burcu Yucel; Associations between cytokine gene polymorphisms and rheumatoid arthritis in Turkish population. Northern Clinics of Istanbul 2020, 7, 563-571, 10.14744/nci.2020.70845.
- Agnieszka Paradowska-Gorycka; Anna Wajda; Katarzyna Romanowska-Próchnicka; Ewa Walczuk; Ewa Kuca-Warnawin; Tomasz Kmiolek; Barbara Stypinska; Ewa Rzeszotarska; Dominik Majewski; Pawel Piotr Jagodzinski; et al.Andrzej Pawlik Th17/Treg-Related Transcriptional Factor Expression and Cytokine Profile in Patients With Rheumatoid Arthritis. Frontiers in Immunology 2020, 11, 0, 10.3389/fimmu.2020.572858.
- Wang P. et al.,; Reduction of follicular regulatory T cells is associated with occurence and development of rheumatoid arthritis. Chin J Cell Mol Immunol. 2021, 37, 152-7.
- Edyta Brzustewicz; Izabella Henc; Agnieszka Daca; Maria Szarecka; Malgorzata Sochocka-Bykowska; Jacek M. Witkowski; Ewa Bryl; Autoantibodies, C-reactive protein, erythrocyte sedimentation rate and serum cytokine profiling in monitoring of early treatment. Central European Journal of Immunology 2017, 3, 259-268, 10.5114/ceji.2017.70968.
- Quentin Simon; Alexis Grasseau; Marina Boudigou; Laëtitia Le Pottier; Eléonore Bettachioli; Divi Cornec; Bénédicte Rouvière; Christophe Jamin; Lucas Le Lann; Maria Orietta Borghi; et al.Rocio Aguilar‐QuesadaYves RenaudineauMarta E. Alarcón‐RiquelmeJacques‐Olivier PersSophie HillionPRECISESADS Clinical ConsortiumPRECISESADS flow cytometry study group A cytokine network profile delineates a common Th1/Be1 pro‐inflammatory group of patients in four systemic autoimmune diseases. Arthritis & Rheumatology 2021, 0, 0, 10.1002/art.41697.
- David Klatzmann; Abul K. Abbas; The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nature Reviews Immunology 2015, 15, 283-294, 10.1038/nri3823.
- Han Sun; Yong Zhao; Kun Wang; Li Zhu; Jian Dong; Jie Zhao; Yimin Wang; Huan Li; Xiaoliang Sun; Yunjie Lu; et al. Low dose IL‐2 suppress osteoclastogenesis in collagen‐induced arthritis via JNK dependent pathway. Immunity, Inflammation and Disease 2020, 8, 727-735, 10.1002/iid3.364.
