Vaccine candidates against COVID-19 have been developed at an unprecedented speed, with more than 200 vaccine candidates currently under investigation. Among those, 20 candidates have entered the clinical Phase 3 to evaluate efficacy, and three have been approved by the European Medicines Agency. The aim of immunization is to act against infection, disease and/or transmission. However, the measurement of vaccine efficacy is challenging, as efficacy trials need to include large cohorts with verum and placebo cohorts. In the future, this will be even more challenging as further vaccine candidates will receive approval, an increasing number of humans will receive vaccinations and incidence might decrease. To evaluate novel and second-generation vaccine candidates, randomized placebo-controlled trials might not be appropriate anymore. Correlates of protection (CoP) could be an important tool to evaluate novel vaccine candidates, but vaccine-induced CoP have not been clearly defined for SARS-CoV-2 vaccines.
- COVID-19
- correlates of protection
- immunogenicity
- SARS-CoV-2
- vaccine
- pandemic
1. Introduction
The ongoing coronavirus disease 2019 (COVID-19) pandemic has caused immense mortality and morbidity, and has also placed huge social and economic burdens on society. At the beginning of 2021, the global case count of infections with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has passed 110 million, with more than 2.5 million confirmed deaths due to the infection [1].
Vaccines can be a key element to limit viral spread. The search for an efficient vaccine has started in January 2020 and progressed at an unprecedented scope, both in the variety of vaccine platforms and in number of candidate vaccines under investigation. One year later, there are more than 250 vaccine candidates in development with 58 having progressed to clinical stages. Detailed lists can be found on various websites, e.g., by the World Health Organization (WHO) [2], London School of Hygiene & Tropical Medicine [3] or the New York Times [4].
In the beginning of 2021, three vaccine candidates have received regular licensure or emergency use authorization, including two mRNA-based and one non-replicating viral vector-based vaccine, in the United States (US), the European Union (EU) and the United Kingdom (UK). A second viral vector vaccine by Janssen (Johnson & Johnson) has been approved by the Food and Drug Administration (FDA) and is awaiting approval by the European Medicines Agency (EMA).
All four vaccines have announced efficacies ranging from 57% to 95% in Phase 3 trials in preventing COVID-19 [5][6][7][8]. Comparable efficacy was demonstrated in different populations in terms of gender, age or ethnicity, while efficacy and safety for populations such as pregnant women or children are still under investigation. Importantly, these four vaccines demonstrated mostly mild to moderate and transient reactogenicity [5][6][7][8]. Of note, it is yet to be determined if the current vaccine candidates are efficacious in reducing or even blocking transmission. A vaccine that confers sterilizing immunity or at least decreases the levels of viral shedding and subsequently infectiousness could significantly impact the containment of SARS-CoV-2.
The duration of immune response to SARS-CoV-2 vaccination remains to be investigated in the upcoming months. Antibody titers induced by natural coronavirus (CoV)-infection have been reported to wane over time. Specifically, human challenge models in past decades have demonstrated the possibility of re-infection with two common cold coronaviruses hCoV-229E and hCoV-OC43 [9][10]. An infection of SARS-CoV-1 or the middle east respiratory syndrome (MERS)-CoV revealed antibody responses that gradually decline over time [11][12]. The current limited data for SARS-CoV-2 infection indicates that humoral immune response persists for at least several months [13][14]. In the light of emerging SARS-CoV-2 variants, most studies assessed protection against the prototype SARS-CoV-2 sequence B.1 or the D614G variant. Whether humoral responses to SARS-CoV-2 may prevent re-infection with novel variants like B1.1.7, B1.315 or P.1 remain unclear to date. First analyses however show reduced neutralization of convalescent plasma in vitro concerning the P.1 or B1.315 variant [15].
Efficacy for the recently approved vaccines has been assessed in randomized placebo-controlled clinical trials (RCT). These trials are considered the gold-standard in clinical research, as they limit the potential for bias in data collection and deliver the highest level of scientific evidence. However, these trials are extremely demanding in terms of resources of any kind. Their implementation may become even more difficult for novel and second-generation vaccine candidates once infection incidence has decreased, or vaccines against SARS-CoV-2 have been properly licensed. With regard to potentially upcoming mutations in the SARS-CoV-2 genome resulting in an escape from human neutralizing antibody (nAb) responses, the introduction of adapted vaccines may be essential to halt the pandemic. RCT might prove to be no longer feasible due to time, cost or ethical reasons. A reasonable alternative would be the measurement of immunity. An immune response that is responsible for and statistically interrelated with protection is named correlate of protection (CoP). The use of CoP may allow the prediction of clinical outcomes (protection against disease or infection) after vaccination or natural infection [16].
CoP can be divided into mechanistic CoP (mCoP), which are causal for protection, and non-mechanistic CoP (nCoP), which are a predictor of protection without being its causal agent [17]. A surrogate is an immune response that substitutes for the true immunological correlate of protection. The identification and measurement of CoP are often challenging. A potential surrogate endpoint for a SARS-CoV-2 vaccine would most likely depend on the characteristics of the vaccine including antigen, method of delivery and method of antigen presentation utilized by the vaccine.
2. Immune Response to SARS-CoV-2
SARS-CoV-2 infection can induce SARS-CoV-2 specific antibodies, CD4+ and CD8+ T-cells (Figure 1 and Figure 2), which target the four structural proteins spike (S), matrix (M), nucleocapsid (N) and envelope (E) as well as to some extent the non-structural proteins [18]. It is assumed that disease severity may critically impact the quality, quantity and duration of immune responses to SARS-CoV-2. Further, numerous reports have discussed the role of nAb and T-cell responses on the severity of the clinical course in COVID-19 [19][20], but there are still knowledge gaps on the broadness, robustness and durability of immune responses. Data on SARS-CoV-2 immunity are emerging rapidly and updates of novel findings are reported by several webpages like the UK biobank report [21].
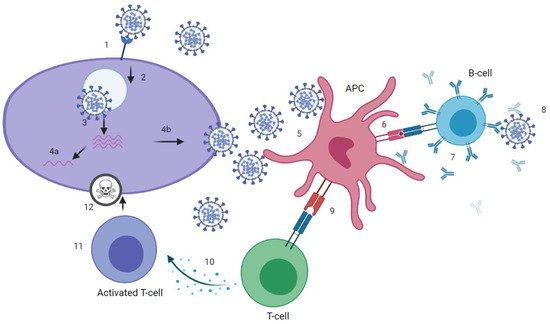
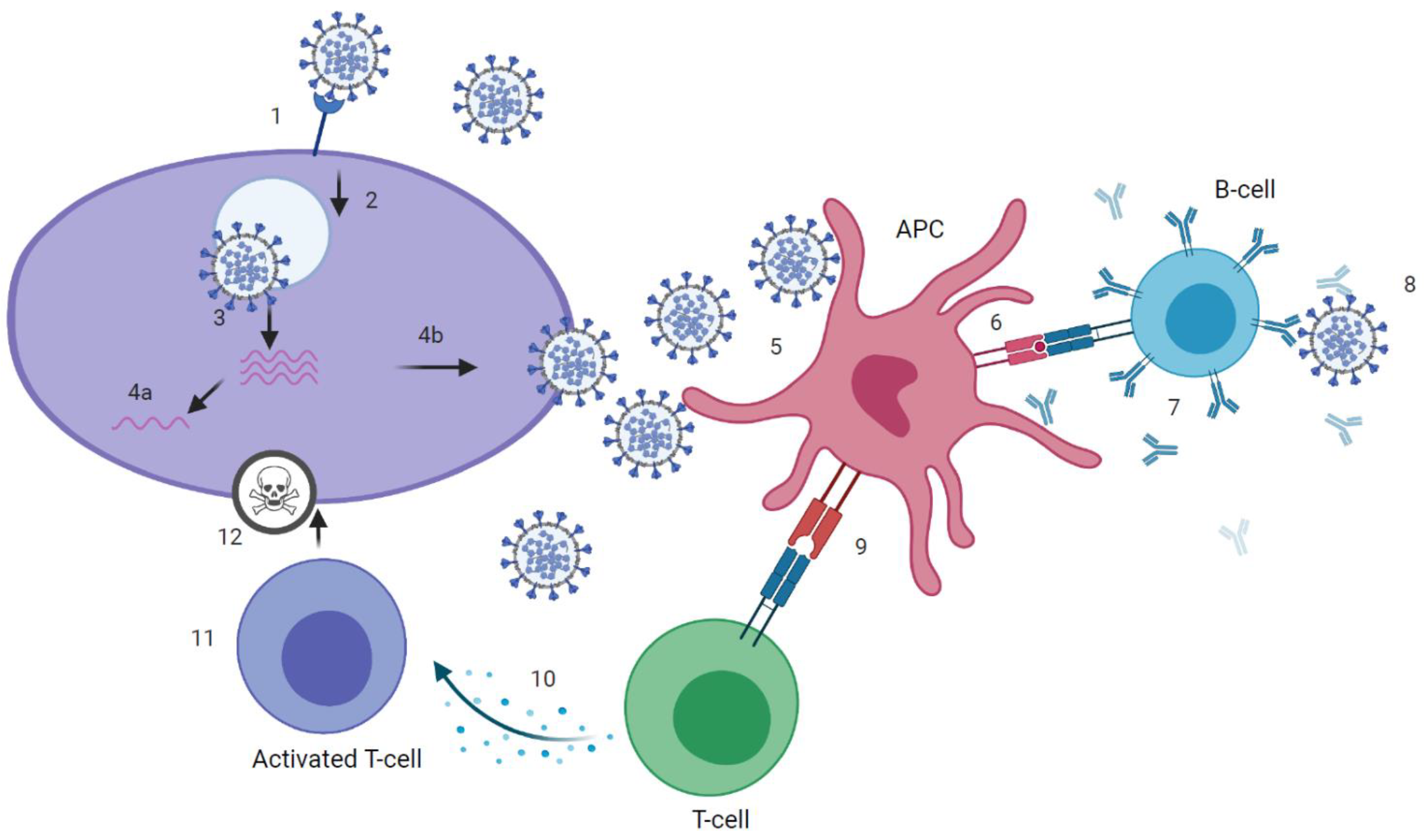
Figure 1.
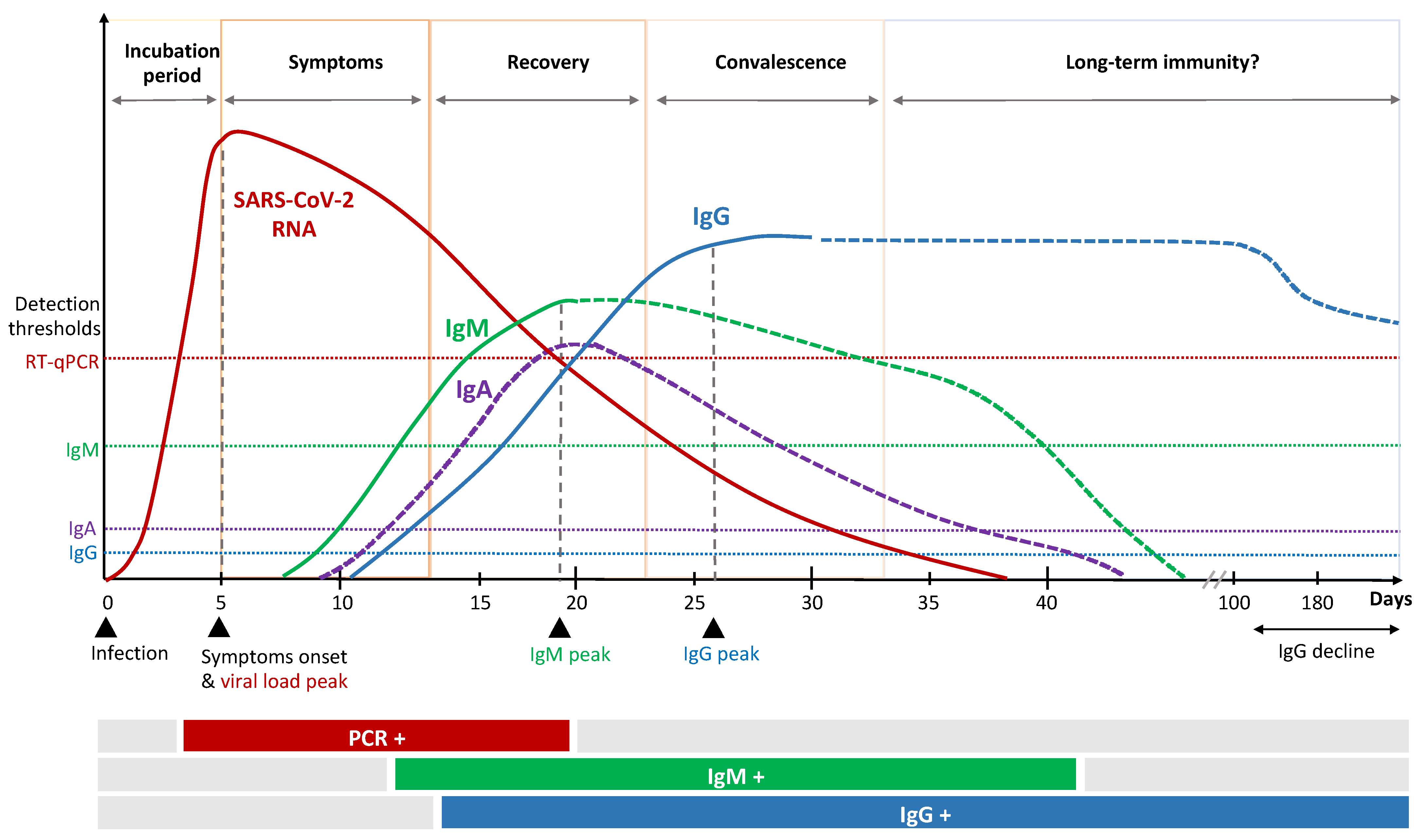
Figure 2. Kinetics of immune response to SARS-CoV-2 infection.
3. Immune Response to SARS-CoV-2 Vaccines
Vaccines against COVID-19 will play a pivotal role for limiting the pandemic. The majority of current vaccine candidates utilize S (or parts thereof like RBD), due to its crucial role in mediating viral entry into cells.
Three vaccine candidates (BioNTech/Pfizer, Moderna & AstraZeneca, Cambridge, UK) have received approval by the EMA, and one is under investigation by the rolling review (Janssen, Belce, Belgium). We here focus on these four forerunner vaccine candidates and delineate their immunogenicity data gained in the framework of Phase 1–3 trials.
The vaccine candidate AZD1222 (Astra Zeneca) is based on a replication deficient chimpanzee adenoviral vector (ChAdOx1) encoding the S protein [7][22]. In a Phase 2/3 trial, volunteers received 5 × 1010 virus particles (vp) as a standard dose for both prime and boost immunization. In addition, a small cohort received a lower dose (2.5 × 1010 vp) as prime immunization. The different study sites used a boost interval that ranged between 4 to 12 weeks. Efficacy in prevention of COVID-19 varied from 62% to 90%, with the variance likely due to heterogeneity in dosing, prime-boost intervals and diversity of study populations. While further studies are ongoing, the vaccine has been approved in the UK with an admitted variance in prime-boost interval between 4 and 12 weeks. The EU has just recently approved the vaccine [23].
Immunogenicity data were reported from a Phase 1/2 trial conducted in the UK [24]. Here, anti-spike IgG responses were observed 28 days after prime with a further increase of titers following boost immunization. Neutralizing antibodies were observed in 91% of participants after prime and 100% after boost immunization in an MNA80 (live SARS-CoV-2 microneutralization) assay. Cellular immunity was also induced by prime and boost immunization, while the boost did not significantly impact the IFN-γ responses (measured with IFN-γ enzyme-linked immunosorbent spot (ELISpot)).
Ad26.COV2.S is another adenovirus-vectored vaccine developed by Janssen. The vaccine is based on a recombinant, replication deficient adenovirus (Ad26) encoding a full-length and stabilized spike protein [25]. In a placebo-controlled phase 1/2a trial, a low dose (5 × 1010 vp) and high dose (1 × 1011 vp) were evaluated as single dose or combined with a booster dose after 56 days. The single dose regimen showed promising results in immunogenicity analyses that warrant further evaluation. After a single vaccination, nAb titers were detected in 90% or more of participants on day 29 and 100% on day 57. Spike-binding antibodies as measured by ELISA correlated well with nAb titers, especially in younger adults. CD4+ T-cell responses on day 14 were induced in 76%–83% of the participants (depending on the dose) with a trend toward type 1 helper T-cells. CD8+ T-cell responses were induced in 51%–64% of participants. Cellular responses were generally lower in higher age groups.
Preclinical data revealed that a single injection resulted in complete protection in lower and upper respiratory tract in rhesus macaques [26]. Clinical data from the Phase 1/2 study also showed induction of T-cell responses and importantly nAb in all participants after a single dose of Ad26.COV2.S (5 × 1010 vp). Janssen therefore initiated a Phase 3 study to evaluate the efficacy of a single dose regimen (clinicaltrials.gov: NCT04505722). By the end of January 2021 a press release was published [8], announcing that the single-shot regime provides efficacy of 57%–72% against moderate to severe COVID-19 and is 85% effective in preventing severe COVID-19. In addition, the efficacy of a two-dose regimen is currently investigated in a parallel trial (clinicaltrials.gov: NCT04614948).
RNA vaccines have been the forerunners in vaccine development against COVID-19. The two RNA-based vaccines developed by BioNTech/Pfizer and Moderna have already received approval from EMA and FDA in December 2020/January 2021. Both are lipid nanoparticle (LNP) formulated nucleoside-modified mRNAs, encoding the stabilized prefusion SARS-CoV-2 spike protein that are administered intramuscularly. Pfizer/BioNTech’s BNT162b2 contains 30 µg of RNA and is administered 21 days apart [5], while Moderna’s mRNA127 contains 100 µg of RNA and is administered by an interval of 28 days [6].
Immunization with BNT162b2 induced binding IgG antibodies against S1 following a single injection, while nAb were detectable in the majority of vaccinees earliest at day 28, seven days following the boost immunization [27]. CD4+ and CD8+ T-cell responses on day 29 were induced in 94.1% (32 out of 34) and 91.9% (34 out of 37) of participants, respectively. T-cell analysis was conducted by an ex vivo IFNy ELISpot assay using stimulation of either CD4+ or CD8+ cells by overlapping peptide pools covering the spike protein. Vaccine efficacy was analyzed in the Phase 3 trial including over 40,000 volunteers that received either BNT162b2 or placebo. Here, the case split of 8 versus 162 COVID-19 cases in the verum and the placebo arm demonstrated a 95% efficacy in prevention of COVID-19 [5].
The mRNA-1273 vaccine by Moderna showed immune responses after the prime injection, with a booster injection resulting in increased titers of both binding and nAb in all participants evaluated in the Phase 1 trial [28]. T-cell responses were analyzed using two pools covering S1 and S2. Here, a Th1-dominant CD4 T-cell response was observed, while CD8+ T-cell responses were low when analyzing responses by intracellular cytokine-staining assay using flowcytometry. In the Phase 3 efficacy trial, over 30,000 volunteers were enrolled and received either mRNA-1273 or placebo. Vaccine efficacy in prevention of COVID-19 was 94.1% with a case split of 11 versus 185 participants in the vaccine and placebo group, respectively [6].
Details on these four forerunner vaccines are listed in Table 1.
Table 1. Overview of forerunner vaccine candidates.
|
Company |
Vaccine (Type) |
Trial (Ref) and NCT |
Humoral Response (Geometric Mean Titer) |
Cellular Response (SARS-CoV-2 Specific) |
|||
|---|---|---|---|---|---|---|---|
|
After 1st Dose |
After 2nd Dose |
CD4 |
CD8 |
||||
|
Pfizer |
BNT162b2 (mRNA expressing spike protein) |
Phase1/2 [29] NCT04380701 |
1:312 (day 7) a |
1:181 (day 85) a |
CD4 T cells in 37/37 pts. (day 7 after boost), in 30/34 de novo response compared to baseline; Th1 > Th2 |
SARS-CoV-2 specific CD8 T cells in 34/37 pts. (91.9%) |
|
|
Moderna |
mRNA-1273 (mRNA expressing spike protein) |
Phase 1 (adults 18 to 55 years) [30] NCT04283461 |
1:4 (day 1) b |
1:654.3 b (day 43) |
CD4 T-cell response Th1 > Th2 |
CD8 T-cell response at low level only after 2nd dose |
|
|
Phase 1 (adults 56 to 70 years and ≥71 years) [31] NCT04283461 |
n/a |
Age 56 to 70 |
CD4 T-cell response Th1 > Th2 in both age groups |
CD8 T-cell response at low level only after 2nd dose |
|||
|
1:402 c and 1:878 d (day 43) |
|||||||
|
Age ≥ 71 |
|||||||
|
1:317 c and 1:317 d (day 43) |
|||||||
|
Phase 3 (interim analysis) [6][32] NCT04470427 |
n/a |
Age 18 to 55 |
CD4 T-cell response Th1 > Th2 in all age groups |
n/a |
|||
|
1:182 c and 1: 430 d (day 119) |
|||||||
|
Age 56 to 70 |
|||||||
|
1: 167 c and 1:269 d (day 119) |
|||||||
|
Age ≥ 71 |
|||||||
|
1:109 c and 1:165 d (day 119) |
|||||||
|
AstraZeneca |
ChAdOx1 nCoV-19 (non-replicating chimpanzee Ad. expressing spike protein) |
Phase 2/3 [33] NCT04400838 |
n/a |
LD/SD (day 42) |
SD/SD (day 42) |
Only available for subgroup (age 18 to 55 years, SD): IFN-γ ELISpot response against SARS-CoV-2 spike protein peaked 14 days after the prime vaccination |
n/a |
|
Age 18 to 55 |
|||||||
|
1:161 e |
1:193 e |
||||||
|
Age 56 to 69 |
|||||||
|
1:143 e |
1:144 e |
||||||
|
Age ≥ 70 |
|||||||
|
1:150 e |
1:161 e |
||||||
|
Janssen |
Ad26.COV2 (recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector encoding a full-length and stabilized SARS-CoV-2 spike (S) protein) |
Phase 1-2a [34] NCT04436276 |
1: 310 (day 57, age 18 to 55 f) |
n/a |
Th1 response to S peptides in - 76% (of low-dose recipients - 83% (of high-dose recipients Th1 > Th2 |
Response detected in - 51% of participants in low-dose group g - 64% in high-dose group g |
|
a Result only reported for 30 µg dose; based on microneutralization assay with a SARS-CoV-2 reporter virus, 50% neutralization titer (VNT50) as readout. b Result only reported for 100 µg dose; based on PRNT80 with authentic SARS-CoV-2. c Based on ID50 pseudovirus neutralization assay. d Based on PRNT80 with authentic SARS-CoV-2. e Based on live SARS-CoV-2 microneutralization assay (MNA80). f Result only reported for 5 × 1010 virus particle single dose. g Identified by expression of INF-γ or IL-2 cytokines on S-peptide stimulation.
Further vaccine candidates are expected to be approved soon, most of them will be administered intramuscularly. While those generally induce systemic immune responses with dominant IgG responses, natural infection induces both systemic and mucosal immune responses [31][32]. The induction of mucosal immune response in the upper respiratory tract generally leads to secretion of secretory IgA, which can be an important factor to induce sterilizing immunity preventing infection and virus transmission [32]. A vaccine candidate that induces mucosal immune response in the upper respiratory tract and thereby potentially sterilizing immunity would be preferable. It has been shown that e.g., application of viral vectors intranasally can lead to strong mucosal immune responses as well as an IgG response [32]. To date, six intranasal and three oral vaccine candidates are in clinical Phase 1 or 2 trials [2]. While data from clinical trials have not yet been published, preclinical studies suggest the induction of mucosal immunity [35]. First results from clinical trials of an oral vaccine candidate by Vaxart Inc. have recently been announced [36].
References
- WHO. WHO Coronavirus Disease (COVID-19) Dashboard. 2021. Available online: (accessed on 2 January 2021).
- WHO. DRAFT Landscape of COVID-19 Candidate Vaccines. World Health Organization. 2020. Available online: (accessed on 2 January 2021).
- LSHTM. London School of Hygiene & Tropical Medicine—COVID-19 Vaccine Tracker 2020. Available online: (accessed on 2 January 2021).
- NYT. Coronavirus Vaccine Tracker. The New York Times, 2 March 2021. Available online: (accessed on 2 January 2021).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615.
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2020, 384, 403–416.
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2020, 397, 99–111.
- Johnson & Johnson. Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial 2021. Available online: (accessed on 2 January 2021).
- Bradburne, A.F.; Somerset, B.A. Coronavirus antibody titres in sera of healthy adults and experimentally infected volunteers. Epidemiol. Infect. 1972, 70, 235–244.
- Callow, K.A.; Parry, H.F.; Sergeant, M.; Tyrrell, D.A.J. The time course of the immune response to experimental coronavirus infection of man. Epidemiol. Infect. 1990, 105, 435–446.
- Liu, W.; Fontanet, A.; Zhang, P.; Zhan, L.; Xin, Z.; Baril, L.; Tang, F.; Lv, H.; Cao, W. Two-Year Prospective Study of the Humoral Immune Response of Patients with Severe Acute Respiratory Syndrome. J. Infect. Dis. 2006, 193, 792–795.
- Payne, D.C.; Iblan, I.; Rha, B.; Alqasrawi, S.; Haddadin, A.; Al Nsour, M.; Alsanouri, T.; Ali, S.S.; Harcourt, J.; Miao, C.; et al. Persistence of Antibodies against Middle East Respiratory Syndrome Coronavirus. Emerg. Infect. Dis. 2016, 22, 1824–1826.
- Gudbjartsson, D.F.; Norddahl, G.L.; Melsted, P.; Gunnarsdottir, K.; Holm, H.; Eythorsson, E.; Arnthorsson, A.O.; Helgason, D.; Bjarnadottir, K.; Ingvarsson, R.F.; et al. Humoral immune response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020, 383, 1724–1734.
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Hastie, K.M.; Yu, E.D.; Faliti, C.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; et al. Immunological memory to SARS-CoV-2 assessed for greater than six months after infection. BioRxiv 2020.
- Madhi, S.A.; Baillie, V.L.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 (AZD1222) Covid-19 vaccine against the B. 1.351 variant in South Africa. MedRxiv 2021.
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065.
- Plotkin, S.A.; Gilbert, P.B. Nomenclature for Immune Correlates of Protection After Vaccination. Clin. Infect. Dis. 2012, 54, 1615–1617.
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1–12.
- Moderbacher, C.R.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 2020, 183, 996–1012.e19.
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. SARS-CoV-2 immunity: Review and applications to phase 3 vaccine candidates. Lancet 2020, 396, 1595–1606.
- UK_Biobank_Limited. UK Biobank 2021. Available online: (accessed on 18 February 2021).
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2020, 396, 1979–1993.
- EMA. EMA Recommends COVID-19 Vaccine AstraZeneca for Authorisation in the EU 2021. Available online: (accessed on 2 January 2021).
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478.
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Marit Groot, A.; Stoop, J.; Tete, S.; van Damme, W.; Leroux-Roels, I.; et al. Safety and immunogenicity of the Ad26. COV2. S COVID-19 vaccine candidate: Interim results of a phase 1/2a, double-blind, randomized, placebo-controlled trial. MedRxiv 2020.
- Mercado, N.B.; Zahn, R.; Wegmann, F.; Loos, C.; Chandrashekar, A.; Yu, J.; Liu, J.; Peter, L.; McMahan, K.; Tostanoski, L.H.; et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020, 586, 583–588.
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. MedRxiv 2020.
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA vaccine against SARS-CoV-2—Preliminary report. N. Engl. J. Med. 2020, 383, 1920–1931.
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585.
- Peng, Y.; Mentzer, A.J.; Liu, G.; Yao, X.; Yin, Z.; Dong, D.; Dejnirattisai, W.; Rostron, T.; Supasa, P.; Liu, C.; et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020, 21, 1336–1345.
- Su, F.; Patel, G.B.; Hu, S.; Chen, W. Induction of mucosal immunity through systemic immunization: Phantom or reality? Hum. Vaccines Immunother. 2016, 12, 1070–1079.
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527.
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422.
- Miorin, L.; Kehrer, T.; Sanchez-Aparicio, M.T.; Zhang, K.; Cohen, P.; Patel, R.S.; Cupic, A.; Makio, T.; Mei, M.; Moreno, E. SARS-CoV-2 Orf6 hijacks Nup98 to block STAT nuclear import and antagonize interferon signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 28344–28354.
- Hassan, A.O.; Kafai, N.M.; Dmitriev, I.P.; Fox, J.M.; Smith, B.; Harvey, I.B.; Chen, R.E.; Winkler, E.S.; Wessel, A.W.; Case, J.B.; et al. A single intranasal dose of chimpanzee adenovirus-vectored vaccine confers sterilizing immunity against SARS-CoV-2 infection. BioRxiv 2020.
- Vaxart. Vaxart Announces Positive Preliminary Data from Phase 1 Clinical Trial Evaluating Its Oral COVID-19 Tablet Vaccine Candidate. Available online: (accessed on 20 February 2021).
