It is confirmed that copper is a self-sanitising metal, acting on human pathogens in a way that does not let them survive exposure to copper or copper alloy surfaces for any reasonable length of time. Regarding the efficacy of copper surfaces, testing in an independent microbiology laboratory has led to 300 various copper surfaces being registered with the United States Environmental Protection Agency (USEPA) in 2008. The registration includes the following statement: “When cleaned regularly, the antimicrobial copper alloy surface kills greater than 99.9% of bacteria within two hours and continues to kill more than 99% of bacteria even after repeated contamination”. This claim acknowledges that copper and its alloys brass and bronze can kill potentially deadly bacteria, and sometime later, it was further understood that copper nanoparticles (Cu-NPs) and laser textured copper also show enhanced antimicrobial activity.
- health care
- infection control
- microbial infections
- Copper
1. A Survey of the Antipathogenic Properties of Copper and Its Alloys
1.1. Copper as an Antibacterial Agent
The contact-killing ability of copper surfaces was studied with respect to bacteria in the early 1980s due to the emerging of hospital superbugs. In 1983, Kuhn [1] compared the bioburden on doorknobs made of brass and stainless steel and found that brass doorknobs showed reduced pathogenic growth in the healthcare environment compared to the stainless steel variety. Recently, Schmidt et al. [2] replaced the normal plastic rails of hospital patients’ beds with copper and then tested for the microbial burden. It was found that the plastic surfaces on the control beds exceeded the recommended amount of bacterial concentrations, but it was not so on the copper beds (94% lower). Salgado et al. [3] installed copper alloys on common touch surfaces in the hospital environment such as bed rails, overbed tables, intravenous poles, arms of visitor’s chairs, nurses’ call buttons, computer mouses, the bezel of the touch screen monitor, and palm rest of laptop computers in three intensive care units (ICU) of three large hospitals in the USA. A total of 650 randomly selected patients were observed in 16 ICU rooms, with eight copper alloy fitted and eight control rooms. The results showed that the MRSA and VRE concentrations were significantly lower (0.071 vs. 0.123;
= 0.020) in the copper-alloy equipped ICUs compared to the standard ICUs [4]. It was also noticed that placing copper alloy surfaces in the ICU rooms reduced the risk of HAI by more than half during the study period, and no HAI outbreak of epidemiologically important organisms occurred in copper-alloy equipped ICUs. A detailed study was conducted in another 16 ICU rooms (eight experimental rooms and eight control rooms) of three hospitals in the USA over 21 months, replacing the normal hand-touch steel surfaces with copper, and this study also found that copper materials at the hand-touch surfaces significantly reduced the microbial burden (698 vs. 6102 CFU per 100 cm
, 88% reduction) [5].
A three-year-long study was conducted in France in five extended care facilities, replacing the doorknobs and handrails with copper alloys. Around 1400 samples were collected and analysed and found that copper doorknobs and handrails revealed significantly less microbial burden (59% and 33% reduction, respectively) than the normal doorknobs and handrails [6]. Other studies conducted in the health care environment have also reported the benefits of replacing plastic hospital beds with copper or copper alloys due to the significant reduction in the microbial burden [7][8][9][10]. However, the studies revealed that the contact-killing property increases with an increase in copper concentration, and a minimum of 60% copper concentration is required in alloys to get the best result [11][5][12][13][14]. Souli et al. [14] studied the antibacterial efficacy of two copper coatings (99% and 63% copper concentrations) on various multi-drug resistant Gram-negative pathogens responsible for nosocomial infections such as
,
spp.,
,
and
They found that copper coatings worked against all strains of the above microbes, with those having greater than 99% copper concentration being able to kill the microbes below six hours (2 h for
, 3 h for
spp., 5 h for
.
, and 6 hr for
.
) [14]. The contact-killing efficacy of copper surfaces on
(a major cause of hospital-acquired infection globally) showed similar characteristics to alloys with higher copper concentrations (>70% copper), killing the
(vegetative cells and spores) after 24–48 h [15]. This microbial contact-killing efficacy of copper has also been found to be successfully applied in wound dressings (Figures 1–3 in [16]).
Apart from the copper concentration, the biocidal efficacy of a surface depends on many other factors such as atmospheric temperature, humidity, length of exposure, microbial type, and concentration [16]. It does seem that contact-killing capability remains high across all standard temperature ranges [17][18]. Noyce et al. [19] studied the characteristics of copper alloys at 22 °C and 4 °C with MRSA and found that at 22 °C, all the three MRSA strains (10
MRSA, EMRSA-1, and EMRSA-16) were completely killed after 45, 60, and 90 min respectively, but it took six hours to completely eradicate these strains at 4 °C [19]. Michels et al. [18] observed a >6.4 log reduction of MRSA when the temperature was 35 °C and humidity was >90%, whereas it was a >6.1 log reduction when the temperature was reduced to 20 °C. It is also noted that the alloys with higher copper concentration (85% and above) were able to completely kill
bacteria at a lower temperature [20]. Similar studies were conducted by Wilks et al. [21][22], who found that antibacterial properties exist at all temperatures but were superior when copper concentrations exceeded 85%. Testing for MRSA at 20 °C on four copper alloys—C19700 (99% Cu), C24000 (80% Cu and 20% Zn), C22000 (90% Cu and 10% Zn), and C77000 (55% Cu, 27% Sn, and 18% Ni)—showed that for C19700, there was a drop off within 75 min and for C22000, drop off was after 270 min. Both are considered to be more than 99% effective [18]. In a similar investigation, Bleichert et al. [23] looked at the biocidal effects of copper surfaces on bacterial and viral biothreat agents and revealed that cells of bacterial biothreat agents exposed to copper surfaces are inactivated within a few minutes. On the other hand, the cells on the control surface (stainless steel) showed a slower decline of the viable cells over time [23].
Whilst most recent studies were in the hospital environment, Inkinen et al. [24] decided to study the antibacterial efficacy of copper in different environmental settings such as retirement homes, kindergartens, and office buildings. Copper replaced traditional materials at the common touch surfaces (such as door handles, light switches, corridor handrails, closet touch surfaces, toilet flush buttons, floor drain lids, and toilet support rails). The study found that the copper surfaces had a lower bacterial load than the reference products and concluded that copper touch surfaces functioned efficiently as an antibacterial surface [24]. It was found that
can form spores and survive on dry surfaces for up to five months, and cannot be killed by hospital-grade disinfectants [25]. However, copper, including its alloys with greater than 70% copper, can kill the
, including the spores [25]. The antimicrobial property of copper regarding
was also studied by Wheeldon et al. [26] in a clinical setting using carrier test methods against dormant and germinating spores and vegetative cells for three hours in the presence and absence of organic matter. It was found that within 30 min, the copper surface destroyed the vegetative cells and reduced the viability of spores exposed to germination within an hour, giving an additional positive signal for using copper in the hospital environment to reduce infection. Besides copper metal and alloys, a copper coating on a steel surface was also found to enhance the antibacterial property of the steel [27].
In the food industry, most bacterial contamination is due to
O157 and is responsible for large-scale food recalls [20]. Noyce et al. [20] studied the efficacy of seven cast copper alloys with copper concentration ranges from 61% to 95% to investigate the ability to reduce
strains in the food industry environment. The study found that without the addition of beef extract, three alloys completely killed the
inoculum within six hours of exposure at 22 °C, but at a lower temperature (4 °C), only the copper alloys with higher copper concentration (>85%) were able to significantly reduce the inoculum [20].
commonly found in soil, water, plant materials, and animals (including humans), are of considerable concern in the food industry [22]. It has been recognised as a human pathogen since 1929, and records show that
infections affect around 2500 people every year in the US, causing 500 deaths annually [22]. It can be critical to pregnant women, the elderly, and immunocompromised people [28]. The bacteria cause Listeriosis, whose symptoms are often septicaemia, encephalitis, spinal meningitis, and corneal ulcers, including pneumonia, which is considered the cause of miscarriage and even death [29][30]. Aisha [31] investigated copper alloys’ antimicrobial effect in killing
and found that copper ions are very effective. Wilks et al. [22] also studied copper’s efficacy in killing
, and found no viable
on any copper alloys after 60 min (5 Log reduction), whereas viable cells were found on stainless steel even after 24 h. Furthermore, they reported that a new alloy called New Silver (65% Cu, 18% Ni, and 17% Zn) also inactivated all bacteria within 90 min of exposure. All these studies support the conclusion that copper products and surfaces can be effectively used in many locations, especially in the health industries and public places, to reduce the bacterial burden and subsequent diseases.
Even though many studies mentioned the influence of copper surfaces in reducing the microbial burden, a review by Cochrane conducted in Australia mentioned that there is only limited evidence available to support the use of environmental fittings with antimicrobial properties in preventing infections with multi-resistant bacterial organisms [32].
1.2. Copper as an Antiviral Agent
The antiviral activity of copper was studied as early as 1958 by Bauer [33], whose work was followed by many researchers who demonstrated the efficacy of copper against many viral strains [34][35][36][37]. Published studies (
and
) confirmed the contact-killing property of copper surfaces against viruses such as influenza virus, norovirus, monkeypox, vaccinia virus, human immunodeficiency virus (HIV), SARS-CoV, and SARS-CoV-2 [38][35][37][33][39]. Researchers at the University of Southampton showed that they could significantly prevent the spreading of influenza using copper surfaces, and it was further revealed that the influenza virus could be eradicated within six hours of exposure to a copper surface [40]. These researchers placed two million active units of influenza A (H1N1) virus on a sheet of copper (C1100, which is pure copper under ISO standards) and stainless steel (S30400) (
). After 24 h, the virus on the steel had declined to 500,000 units, but only 500 viruses were found to be active after six hours on the copper [41][40][42].
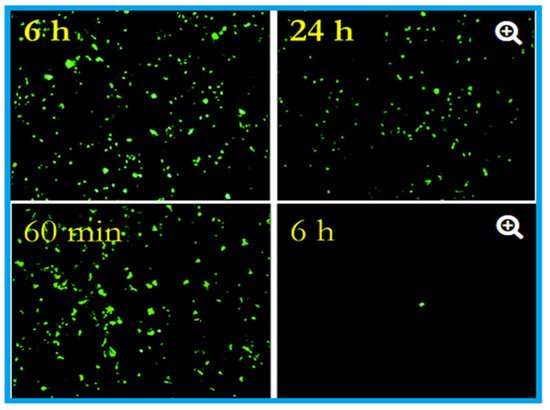
Effects of influenza A virus on steel surface (top) and copper surface (bottom). The influenza virus was cultured in R-mix vials that contain a monolayer of mink lung and human laryngeal carcinoma cells grown on glass coverslips. This was inoculated into sterile coupons of copper (C11000) and steel (20 µL virus suspension with 10
virus particles per millilitre) for the experimental purpose and kept at room temperature (22 ± 2 °C) with a relative humidity of 50 to 60%. Here in the epifluorescent image, the number of green fluorescing cells are proportional to the viral inoculum. After six hours, 10
virus particles were found to be remained viable on the steel surface, and after 24 h, 5 × 10
particles were present, capable of causing cell infection (top). In contrast to the steel surface, on copper, the virus particles reduce to 5 × 10
after 60 min (the equivalent of 24 h of exposure on stainless steel), which reduced to 5 × 10
after six hours (nearly 4 log reduction). After 24 h of incubation, 500,000 virus particles were present on stainless steel, but 500 only seen after six hours on the copper surface. Adapted with permission from Ref. [42]. Copyright 2007, American Society for Microbiology.
Warnes et al. [43] tested the capability of inactivating one corona group virus, (229E), that can cause common colds and pneumonia. They found that the virus became inactivated immediately after being kept on copper, but it stayed viable for five days on stainless steel and glass (
). Similar to bacteria, inoculation efficiency for the virus also depends on temperature, humidity, copper concentration [38][19][20][21][43][44][45], length of exposure, and microbial density [19][21][22][46].
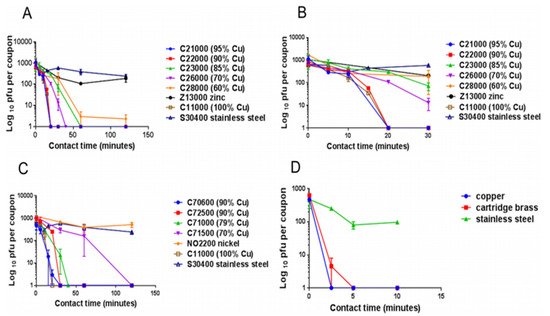
The panels describe the rapid contact-killing efficiency of various copper alloys (varies from 60% copper concentration to 100%) on human coronavirus-229E (HuCoV-229E), which causes the common cold. Initially, around 10
PFU HuCoV-229E (20 µL infected cell lysate) was applied on one sq. cm copper alloy coupon(s) with various copper concentrations, including stainless steel, nickel, and zinc (as control metals). (
) It was found that the coronavirus was inactivated less than 40 min on brass coupons and less than 120 min on copper-nickel alloy (containing less than 70% copper). Surprisingly the alloy with 70% copper showed quick antiviral activity than the alloy with 85% copper. (
) The observation showed an initial time lag on all alloys and metals, followed by rapid inactivation on copper coupons. The control metals stainless steel and nickel did not show any anti-coronavirus activity, except zinc, which showed little (significant only after 60 min,
= 0.046). (
) When the copper concentration was reduced to 70%, it took approximately 80 min more to inactivate all the viruses compared to one with 79% copper. (
) When the same inoculum was applied at 1 µL per sq. cm, the coupons inactivated the virus eight-time faster. The experiment showed that the concentration of copper and the amount of virus are significant factors in showing the antiviral activity. Adapted from Ref. [43].
Norovirus is highly infectious, causing viral gastroenteritis, and is spread through touch surfaces [47]. Warnes and Keevil [39] investigated the antiviral property of copper alloy surfaces against norovirus and found its effectiveness is proportional to the copper concentration in the alloy. Furthermore, they observed that antiviral effectiveness was not very rapid on brass but was very effective on the copper-nickel alloy. It is also found that copper-based filters inactivate HIV-1, which can significantly reduce HIV-1 infection through breastfeeding and blood donation [48]. In addition to this, Noyce et al. [42] indicated that copper surfaces act as a barrier against the avian flu epidemic. Their experiments have shown that, after six hours of exposure on a copper surface, 99.9% of the two million active H5N1 virus particles involved in the experiment became inactive.
Details of contact-killing or inactivation of microbes by copper surfaces. Adapted with permission from Ref. [38]. Copyright 2007, American Society for Microbiology.
| Species | Application Method (Wet (W)/Dry (D)) |
Time to No Viable Forms Detected | Reference(s) |
|---|---|---|---|
| SARS-CoV-2 | D, 105.25 50% (TCID50) per mm | 4 h | [51][37] | |||
| SARS-CoV | ||||||
| SARS-CoV | The virus was active only up to 8 h on the copper surface | D, 106.75–7.00 TCID50[51][37] | ||||
| /mm | 8 h | [ | 51][37] | Influenza A virus | After incubation for six hours on copper 99% of the viral particles were inactivated | [ |
| Human coronavirus—HCoV-229E | 92 | ] | W, 103[42] | |||
| PFU | 20 min | [ | 93][43] | Influenza A virus | Solid-state copper oxide (Cu2O) inactivated the influenza A virus | [99][49] |
| Influenza A virus (H1N1) | W, 5 × 105 viruses h | 5 h | [92][42] | Human coronavirus HuCoV-229E |
Active only 20 min on copper surface | [93][43] |
| Penicillium crysogenum | W, (2–300) × 105 spores c | 24 h | [9][62] | Hepatitis C virus (HCV) |
Copper oxide-NPs significantly inhibit the infectivity of HCV, both at the entry and attachment stages | |
| Fusarium solani | W, (2–300) × 105 spores c | [100][50] | ||||
| 24 h | [ | 9 | ][62] | Murine norovirus-1 (MNV-1) |
Copper alloy (65 to 99.9% Cu) dry surfaces inactivated the MNV-1 | |
| Fusarium oxysporum | W, (2–300) × 105 spores | [90][39] | ||||
| c | 24 h | [ | 9][62] | Vesicular Stomatitis Virus Coxsackie Virus-B4 Respiratory Syncytial Virus |
Curcumin-copper synthesised compound found to effective against these viruses and could be utilised for the development of vaginal microbicidal gel | [101][51] |
| Feline Calicivirus | ||||||
| Fusarium culmonium | W, (2–300) × 105 spores c | 24 h | [9][62] | (FCV) |
CuI-NPs reduced the infectivity of FCV by order of seven magnitude | |
| Aspergillus niger | W, (2–300) × 105 spores c | [102][52] | ||||
| >576 h | [ | 9 | ][62] | H1N1 Influenza Virus 2009 Pandemic |
CuI-NPs showed antiviral activity against influenza A virus of swine-origin | [10][35] |
| Human Immunodeficiency Virus-1 (HIV-1) |
When exposed to copper oxide, the HIV-1 infectivity inhibited in a dose-dependant manner |
[98][48] | ||||
| Polio Virus | Copper sulphate (20 mg/L) completely inactivated the polio virus in the presence of hydrogen peroxide | [103][53] | ||||
| Herpes Simplex Virus (HSV) |
Reducing agents such as ascorbic acid, hydrogen peroxide and cysteine enhanced the antiviral property of copper | [104][54] |
1.3. Copper as an Antifungal Agent
The antifungal property of copper was first identified in 1761 when it was found that grain seeds soaked in copper sulphate solutions could inhibit the seed-borne fungi, but it took more than 100 years for the more sophisticated development of the fungicide “Bordeaux mixture” (developed by Pierre-Marie-Alexis Millardet and used in the USA) and “Burgundy mixture” (used in France) [55]. Copper sulphate and lime mixtures were sprayed onto grape wines to make them mildew-free, prevent fungal infection in other plants, and control the algal growth in water reservoirs as well on timber, and were also found useful in preserving fabric [55]. This experience again shows that even though the antimicrobial property of copper has been used in the agriculture sector for controlling fungal and bacterial infections for many years [56][57], it has come to the healthcare environment very lately.
A peer-reviewed study of the fungicidal property of copper was carried out in the 1950s, finding that copper, including copper compounds, are effective in killing several fungi and yeast, including
[57], and
[60]. Indeed, many thousand tons of copper-based antifungal agents, specifically copper sulphate and copper hydroxide, are annually used across the globe for agricultural purposes [61]. It is also used in wood processing to prevent roof moss formation and as an algae-resistant roofing system in the 3M industry. The biocidal efficacy of copper against
and
species as well as
and
was studied by Weaver et al. [62], who found that copper surfaces were able to kill most of these fungi and were able to prevent germination of new spores. The mechanism for control with bacteria and fungi is similar as inoculation starts with membrane damage, followed by enlargement and disappearance of vacuoles and the onset of oxidative stress.
spp. can commonly survive in the healthcare environment and can cause HAIs [63]. The efficacy of copper-sputtered polyester surfaces (Cu-PES) was tested against azole-resistant
and
under dark and low-intensity visible light, with the results showing that under low-intensity visible light, the Cu-PES exhibited fungicidal activity against both strains within 30 min of exposure [63]. Of interest, it was found that, in addition to the pure copper surfaces, many copper compounds, such as the copper (II) complex of quinoline-2, could act as antifungal agents [64]. Ghasemian et al. [65] tested the antifungal efficiency of Cu-NPs against filamentous fungi (
and
and found that Cu-NPs are very effective control agents, finding that particle size is a significant factor in antimicrobial activity. Two other studies also found that Cu-NPs are effective against
species [66][67]. The contact-killing ability of copper for various microbes, including fungi, is summarised in
.
| Aspergillus fumigatus | |||
| W, (2–300) × 10 | |||
| 5 | |||
| spores | |||
| c | |||
| >120 h | |||
| [ | 9 | ] | [62] |
| Aspergillus flavus | W, (2–300) × 105 spores c | 120 h | [9][62] |
| Candida albicans | W, 105 CFU f | 1 h | [59][68] |
| Saccharomyces cerevisiae | D, 106 CFU k | 30 s | [117][69] |
| Candida albicans | D, 106 CFU k | 5 min | [117][69] |
| Candida albicans | W, (2–300) × 105 spores c | 24 h | [118][70] |
| MRSA d | W, 107 CFU f | 3 h | [59][68] |
| MRSA NCTC 10442 | W, 2 × 107 CFU | 75 min | [75][18] |
| EMRSA-16 e (NCTC13143) | W, (1–1.9) × 105 CFU c | 90 min | [74][17] |
| EMRSA-1 e (NCTC11939) | W, (1–1.9) × 107 CFU c | 1 h | [74][17] |
| MRSA d (NCTC10442) | W, (1–1.9) × 107 CFU c | 45 min | [74][17] |
| Acinetobacter baumannii | W, 107 CFU f | 3 h | [59][68] |
| Pseudomonas aeruginosa | W, 107 CFU f | 3 h | [59][68] |
| Klebsiella pneumoniae | W, 107 CFU f | 1 h | [59][68] |
| Mycobacterium tuberculosis | W, 2.5 × 107 CFU f | 5–15 days | [59][68] |
| C. difficile (ATCC 9689) vc&spores | W, 2.2 × 105 CFU c | 24–48 h | [73][15] |
| Pseudomonas aeruginosa PAO1 | W, 2.2 × 107 CFU j | 2 h | [74][17] |
| Escherichia coli O157 | W, 2.7 × 107 CFU c | 75 min | [75][18] |
| Listeria monocytogenes Scott A | W, 107 CFU c | 1 h | [77][20] |
| Escherichia coli O157 | W, (3–4) × 107 CFU c | 65 min | [78][21] |
| Brucella melitensis NCTC 10094 | D, 106 CFU k | <5 min | [80][23] |
| Burkholderia mallei NCTC 3709 | D, 106 CFU k | <5 min | [80][23] |
| Burkholderia pseudomallei NCTC 0816-03 | D, 106 CFU k | <5 min | [80][23] |
| Francisella tularensis FSC 237 | D, 106 CFU k | <5 min | [80][23] |
| Yersinia pestis NCTC 2028 | D, 106 CFU k | <5 min | [80][23] |
| C. difficile germinating spores | W, 8 × 106 CFU i | 3 h | [82][26] |
| C. difficile dormant spores | W, 8 × 106 CFU i | ua-3 h | [82][26] |
| C. difficile NCTC11204/R20291 vc | W, (1–5) × 106 CFU i | 30 min | [82][26] |
| Different Enterococcus spp. | W, 106 CFU f | 1 h | [96][46] |
| Enterococcus hirae ATCC 9790 | W, 107 CFU c | 90 min | [96][46] |
| Escherichia coli W3110 | D, 109 CFU k | 1 min | [119][71] |
| Brachybacterium conglomeratum DSM10241 | D, 109 CFU k | A few min | [119][71] |
| Staphylococcus warneri DSM 20316 | D, 109 CFU k | A few min | [119][71] |
| Pseudomonas oleovorans DSM1045 | D, 109 CFU k | 1 min | [119][71] |
| Pantoea stewartii DSM30176 | D, 109 CFU k | 1 min | [119][71] |
| Acinetobacter johnsoni SM6963 | D, 109 CFU k | 1 min | [119][71] |
| Campylobacter jejuni | W, 4.5 × 106 CFU b | 8 h | [120][72] |
| Salmonella enterica | W, 4.5 × 106 CFU b | 4 h | [120][72] |
2. Application of Copper Nanoparticles (Cu-NPs)
Nanotechnology is attracting global attention due to its enormous potential in a wide range of applications, and Cu-NPs have attracted more significance both in the health and food industries because of their antimicrobial characteristics. Various methods are used for the preparation of Cu-NPs, such as microwave irradiation, thermal reduction, vacuum vapour deposition, chemical reduction, laser ablation, and polyol [72]. Remyadevi et al. [73] synthesized Cu-NPs using the modified polyol method and carried out the antimicrobial activity against several bacteria (
and
) and fungi (
and
). After careful study, they revealed that Cu-NPs exhibit antimicrobial activity, which is strong in bacteria than the fungi [73]. Apart from this, it is noted that Cu-NPs showed antimicrobial property against MRSA,
,
,
.
serotype Choleraesuis (S. Choleraesuis) [66][74], and hepatitis C [50]. After a detailed study and data analysis, Raffi et al. [75] concluded that Cu-NPs with a large surface-to-volume ratio efficiently inactivate
bacteria.
The Cu-NPs can be immobilized and coated onto various surfaces to generate or improve antimicrobial activity. In this respect, Cu-NPs- and nanoparticle-impregnated materials, including cloths and plastic, have been shown to exhibit antimicrobial properties, which can be used in various fields, specifically in the health industry [76]. For example, some researchers found that Cu-NP-impregnated face masks showed biocidal activity against human and avian influenza A virus [48].
As mentioned above, the antimicrobial properties of Cu-NP fabrics have been incorporated in textile technology to develop materials suited for use in the health industry, and a specific platform technology has been developed to introduce copper into cotton fibres, latex, and other polymeric materials. The copper oxide NPs (CuO-NPs) (3%–10%) (prepared by a wet chemical method) were microencapsulated by ionic gelation and applied to plain weave cotton fabric, following a pad-dry-cure technique, and it was found that the fabric demonstrated a high level of antimicrobial activity, which could be used in the healthcare environment to reduce the bioburden [77]. In this respect, the studies of Niiyama et al. [78] and Palza et al. [79] are very encouraging. Niiyama et al. [78] determined whether a copper film bedsheet would reduce MRSA infection in a dermatology ward and found that the MRSA count on the sheet coated with Cu-NPs was significantly lower (20–30 colony-forming units, or CFUs) when compared to the non-copper coated bed sheet (6600–11,000 CFUs). Palza et al. [79] tested the antimicrobial behaviour of materials with copper-based additives (copper NPs on plastic matrices) in a hospital environment. As a part of this study, the researchers replaced the normal plastic waiting room chairs with Cu-NPs-embedded chairs, and IV poles made of metals were coated with organic paint impregnated with nanostructured zeolite/copper. They continued sampling once a week for ten weeks and analysed the levels of viable microorganisms. It was found that the copper substrates reduced 73% of the viable microbes in the waiting room chairs and found only low levels of microbes remaining in the IV poles. Apart from this, Harikumar and Aravind [77] investigated the antimicrobial characteristics of Cu-NPs and Cu-nanocomposites against
using the well diffusion method and found that the antimicrobial activity increases with an increase in particle dose and contact time.
Research indicates that the antibacterial activity of copper-based NPs is far superior to that of a normal copper surface, mainly because of the small size (high surface area compared to the volume) and higher cell penetration [80]. Several studies indicated that both Cu-NPs and copper oxide (Cu
O) induced DNA degradation occurs in Gram-positive and negative bacteria even though the concentration of released ions was far below the normal level for inhibiting bacterial growth [80][81]. This highlighted that with Cu-NPs, the concentration of released ions is less significant than the effect of NP size [81]. Other studies support this finding and have suggested that the size of the Cu-NPs is the major contributing factor for its antimicrobial activity [66][82]. To support this, Padil and Cernik [83] found that small (4.8 +/−1.6 nm) CuO-NPs have significantly higher antimicrobial activity than the larger particles (7.8 +/−2.3 nm), whilst Applerot et al. [80] surmised that the advantage of small particles would be their higher penetration capacity into the microbial covering. In addition to their antibacterial activity, normal copper and Cu-NPs were shown to have antifungal property against several fungi, including C.
and
(
) [65][69], and found that the control mechanism in fungi was similar to that in the case of bacteria [69].
Currently, Cu-NPs are used in various industries and production sectors, but this increased use comes with a cost. Copper NPs have several adverse effects regarding environmental health since, in many regions, these copper NPs have been released into the environment. This is of special concern in the aqueous environment, where it generates health risks to aqueous organisms. It has been observed that copper NPs are not only toxic to bacteria but act on some species of fish and also on mice [84][85][86]. In general, copper, which is released from various industries, normally emerges in two forms, either as dissolved copper or particulate-bound copper, and the toxicity and the mobility of the copper depend on the form [87].
3. The Various Applications of Copper in the Built Environment
Cooling towers and potable water distribution systems have recently been determined as the source of hospital outbreaks of several Legionnaires’ diseases [135,136]. However, after several studies, it has been confirmed that the use of a copper-silver ionisation system is the most successful long-term water disinfection system that can be used in the hospital environment [137,138,139,140,141]. In similar investigations, it has been established that in the dental industry, dental cement having copper shows potential antimicrobial properties [142], and in the food industry environment, shifting to copper surfaces and copper-made food carrying, and transportation surfaces produce a significant reduction in foodborne diseases [77,78,120,121,132,143,144]. In addition, thin-film copper oxide (CuO)-coated glass [145], CuO-impregnated degradable phosphate glass fibres [146], and copper alloys also have shown potential biocidal properties against string bacteria spores [73,145]. Drinking glasses made of copper or copper-impregnated glasses were found to reduce the biofilm formation, thereby reducing the risk of several potential infections [143]. Copper and copper alloys can also be used to produce sanitary installation tubes, fittings, door handles, knobs, hand-rails, and vehicle door handles to reduce the microbial burden.Cooling towers and potable water distribution systems have recently been determined as the source of hospital outbreaks of several Legionnaires’ diseases [88][89]. However, after several studies, it has been confirmed that the use of a copper-silver ionisation system is the most successful long-term water disinfection system that can be used in the hospital environment [90][91][92][93][94]. In similar investigations, it has been established that in the dental industry, dental cement having copper shows potential antimicrobial properties [95], and in the food industry environment, shifting to copper surfaces and copper-made food carrying, and transportation surfaces produce a significant reduction in foodborne diseases [20][21][72][73][85][96][97]. In addition, thin-film copper oxide (CuO)-coated glass [98], CuO-impregnated degradable phosphate glass fibres [99], and copper alloys also have shown potential biocidal properties against string bacteria spores [15][98]. Drinking glasses made of copper or copper-impregnated glasses were found to reduce the biofilm formation, thereby reducing the risk of several potential infections [96]. Copper and copper alloys can also be used to produce sanitary installation tubes, fittings, door handles, knobs, hand-rails, and vehicle door handles to reduce the microbial burden.
Apart from the above, silver-zinc (Ag-Zn) and silver-copper (Ag-Cu) incorporated soda-lime glass prepared by ion exchange has shown significant antimicrobial properties [147], which could be useful for several daily applications, specifically in the health industry and public places. Copper metal and CuO-NPs embedded in a polypropylene matrix is also an evidence of antimicrobial property. After careful preparation, Delgado et al. [148] found that the composite has a strong antimicrobial activity againstApart from the above, silver-zinc (Ag-Zn) and silver-copper (Ag-Cu) incorporated soda-lime glass prepared by ion exchange has shown significant antimicrobial properties [100], which could be useful for several daily applications, specifically in the health industry and public places. Copper metal and CuO-NPs embedded in a polypropylene matrix is also an evidence of antimicrobial property. After careful preparation, Delgado et al. [101] found that the composite has a strong antimicrobial activity against
E. coliand could kill 95% of the colony within four hours, acting through the release of the Cu
2+ions.
Although many water purification methods exist, potable water is still beyond the reach of millions of people around the globe. Sudha et al. [149] conducted a study to investigate the microbial efficacy of copper pots in killing the bacteria (Although many water purification methods exist, potable water is still beyond the reach of millions of people around the globe. Sudha et al. [102] conducted a study to investigate the microbial efficacy of copper pots in killing the bacteria (
V. choleraO1,
Shigella flexneri (S. flexneri)2a,
E. coli, Salmonella enterica Typhi (S. Typhi),and
Salmonella enterica serovar Paratyphi (S. Paratyphi) in the water. When drinking water was contaminated with 500 CFU/mL of those bacteria stored in copper pots for 16 h at room temperature, no bacteria were recovered in the culture medium. They observed a slight alteration in the water pH from 7.83 to 7.93, but no other changes were observed. Copper pots are consequently considered an interim microbial purification solution for drinking water in under-developed and many developing country areas [149]. It is also found that a copper-coiled device put into a glass bottle overnight can also inactivate the bacteria, including) in the water. When drinking water was contaminated with 500 CFU/mL of those bacteria stored in copper pots for 16 h at room temperature, no bacteria were recovered in the culture medium. They observed a slight alteration in the water pH from 7.83 to 7.93, but no other changes were observed. Copper pots are consequently considered an interim microbial purification solution for drinking water in under-developed and many developing country areas [102]. It is also found that a copper-coiled device put into a glass bottle overnight can also inactivate the bacteria, including
E. coli,
S. typhi,and V.
cholera [150]. This observation still needs to be investigated for the possibility of viable but non-culturable microbial inactivation. It is recalled that the use of copper or copper alloy pots were common in Indian, Chinese, and Egyptian households, but they were displaced by the arrival of cheaper aluminium, steel, and plastic wares. The major advantage of copper’s biocidal activity is that there is no need for any kind of energy, fuel, including electricity. It is also found that similar to dry copper metal efficacy, the biocidal effectiveness of copper in water also follows a temperature pattern, with the fastest biocidal effects occurring at higher temperatures [151]. The presence of chloride salts (NaCl) resulted in faster inactivation (of[103]. This observation still needs to be investigated for the possibility of viable but non-culturable microbial inactivation. It is recalled that the use of copper or copper alloy pots were common in Indian, Chinese, and Egyptian households, but they were displaced by the arrival of cheaper aluminium, steel, and plastic wares. The major advantage of copper’s biocidal activity is that there is no need for any kind of energy, fuel, including electricity. It is also found that similar to dry copper metal efficacy, the biocidal effectiveness of copper in water also follows a temperature pattern, with the fastest biocidal effects occurring at higher temperatures [104]. The presence of chloride salts (NaCl) resulted in faster inactivation (of
E. coli) compared with pure water, but the presence of complex organic mixtures such as humic acids, proteins, and amino acids reduced the inactivation [151]. It gives an indication that natural organic constituents and salts in the water influence the antibacterial efficacy of copper when used to treat water.) compared with pure water, but the presence of complex organic mixtures such as humic acids, proteins, and amino acids reduced the inactivation [104]. It gives an indication that natural organic constituents and salts in the water influence the antibacterial efficacy of copper when used to treat water.
In addition to the above, the antimicrobial efficacy of copper-impregnated textiles and latex were mentioned in many studies [152,153]. For example, research from Israel highlighted the antimicrobial properties of copper-impregnated textiles [152], and the researchers developed copper-impregnated textile products such as cotton and latex and tested them against various bacteria (In addition to the above, the antimicrobial efficacy of copper-impregnated textiles and latex were mentioned in many studies [105][106]. For example, research from Israel highlighted the antimicrobial properties of copper-impregnated textiles [105], and the researchers developed copper-impregnated textile products such as cotton and latex and tested them against various bacteria (
E. coli, S. aureus, MRSA, and VRE), viruses (HIV-1 and West Nile virus), and fungi (
C. albicans). They demonstrated that >2 log reduction of all tested bacteria occurred within two hours, and the materials inactivated the fungi within an hour. They also observed that latex gloves were able to reduce the infectivity of both viruses (HIV-1 and West Nile virus). Later studies also found that copper oxide-impregnated fabrics could reduce >98.7% of microbes within 20 to 240 min of exposure [153].). They demonstrated that >2 log reduction of all tested bacteria occurred within two hours, and the materials inactivated the fungi within an hour. They also observed that latex gloves were able to reduce the infectivity of both viruses (HIV-1 and West Nile virus). Later studies also found that copper oxide-impregnated fabrics could reduce >98.7% of microbes within 20 to 240 min of exposure [106].
References
- Kuhn, P.J. Doorknobs: A source of nosocomial infection. Diagn. Med. 1983, 6, 62–63.
- Schmidt, M.G.; Attaway, H.H.; Fairey, S.E.; Howard, J.; Mohr, D.; Craig, S. Self-disinfecting copper beds sustain terminal cleaning and disinfection effects throughout patient care. Appl. Environ. Microbiol. 2019, 86, e01886-19.
- Salgado, C.D.; Sepkowitz, K.A.; John, J.F.; Cantey, J.R.; Attaway, H.H.; Freeman, K.D.; Sharpe, P.A.; Michels, H.T.; Schmidt, M.G. Copper surfaces reduce the rate of healthcare-acquired infections in the intensive care unit. Infect. Control Hosp. Epidemiol. 2013, 34, 479–486.
- OECD. Healthcare-associated infections. In Health at a Glance: Europe 2018: State of Health in the EU Cycle, Organisation of Economic Cooperation and Development; OECD Publishing: Brussels, Belgium, 2018.
- Schmidt, M.G.; Attaway, H.H.; Fairey, S.E.; Steed, L.L.; Michels, H.T.; Salgado, C.D. Copper continuously limits the concentration of bacteria resident on bed rails within the ICU. Infect. Control Hosp. Epidemiol. 2013, 34, 530–533.
- Colin, M.; Klingelschmitt, F.; Charpentier, E.; Josse, J.; Kanagaratnam, L.; Champs, C.D.; Gangloff, S.C. Copper alloy touch surfaces in healthcare facilities: An effective solution to prevent bacterial spreading. Materials 2018, 11, 2479.
- Mikolay, A.; Huggett, S.; Tikana, L.; Grass, G.; Braun, J.; Nies, D.H. Survival of bacteria on metallic copper surfaces in a hospital trial. Appl. Microbiol. Biotechnol. 2010, 87, 1875–1879.
- Efstathiou, P. The Role of Antimicrobial Copper Surfaces in Reducing Healthcare-Associated Infections. Eur. Infect. Dis. 2011, 5, 125–128. Available online: (accessed on 17 October 2020).
- O’gorman, J.; Humphreys, H. Application of copper to prevent and control infection. Where are we now? J. Hosp. Infect. 2012, 81, 217–223.
- von Dessauer, B.; Navarrete, M.S.; Benadof, D.; Benavente, C.; Schmidt, M.G. Potential effectiveness of copper surfaces in reducing health care–associated infection rates in a pediatric intensive and intermediate care unit: A nonrandomized controlled trial. Am. J. Infect. Control 2016, 44, e133–e139.
- Schmidt, M.G.; Attaway, H.H.; Sharpe, P.A.; John, J., Jr.; Sepkowitz, K.A.; Morgan, A.; Fairey, S.E.; Singh, S.; Steed, L.L.; Cantey, J.R.; et al. Sustained reduction of microbial burden on common hospital surfaces through the introduction of copper. J. Clin. Microbiol. 2012, 50, 2217–2223.
- Schmidt, M.G.; Dessauer, B.v.; Benavente, C.; Benadof, D.; Cifuentes, P.; Elgueta, A.; Duran, C.; Navaratte, M.S. Copper surfaces are associated with significantly lower concentrations of bacteria on selected surfaces within a paediatric intensive care unit. Am. J. Infec. Control 2016, 44, 203–209.
- Zhu, L.; Elguindi, J.; Rensing, C.; Ravishankar, S. Antimicrobial activity of different copper alloy surfaces against copper resistant and sensitive Salmonella enterica. Food Microbiol. 2012, 30, 303–310.
- Souli, M.; Antoniadou, A.; Katsarolis, I.; Mavrou, I.; Paramythiotou, E.; Papadomichelakis, E.; Drogari-Apiranthitou, M.; Panagea, T.; Giamarellou, H.; Petrikkos, G.; et al. Reduction of environmental contamination with multidrug-resistant bacteria by copper-alloy coating of surfaces in a highly endemic setting. Infect. Control Hosp. Epidemiol. 2017, 38, 765–771.
- Weaver, L.; Michels, H.T.; Keevil, C.W. Survival of Clostridium difficile on copper and steel: Futuristic options for hospital hygiene. J. Hosp. Infect. 2008, 68, 145–151.
- Arendsen, L.P.; Thakar, R.; Bassett, P.; Sultan, A.H. The use of copper as an antimicrobial agent in health care, including obstetrics and gynecology. Clin. Microbiol. Rev. 2019, 32, e00125-18.
- Elguindi, J.; Wagner, J.; Rensing, C. Genes involved in copper resistance influence survival of Pseudomonas aeruginosa on copper surfaces. J. Appl. Microbiol. 2009, 106, 1448–1455.
- Michels, H.T.; Noyce, J.O.; Keevil, C.W. Effects of temperature and humidity on the efficacy of methicillin—Resistant Staphylococcus aureus challenged antimicrobial materials containing silver and copper. Appl. Microbiol. 2009, 49, 191–195.
- Noyce, J.O.; Michels, H.; Keevil, C.W. Potential use of copper surfaces to reduce survival of epidemic methicillin-resistant Staphylococcus aureus in the healthcare environment. J. Hosp. Infect. 2006, 63, 289–297.
- Noyce, J.O.; Michels, H.; Keevil, C.W. Use of copper cast alloys to control Escherichia coli O157 cross-contamination during food processing. Appl. Environ. Microbiol. 2006, 72, 4239–4244.
- Wilks, S.A.; Michels, H.; Keevil, C.W. The survival of Escherichia coli O157 on a range of metal surfaces. Int. J. Food Microbiol. 2005, 105, 445–454.
- Wilks, S.A.; Michels, H.T.; Keevil, C.W. Survival of Listeria monocytogenes Scott A on metal surfaces: Implications for cross-contamination. Int. J. Food Microbiol. 2006, 111, 93–98.
- Bleichert, P.; Santo, C.E.; Hanczaruk, M.; Meyer, H.; Grass, G. Inactivation of bacterial and viral biothreat agents on metallic copper surfaces. Biometals 2014, 27, 1179–1189.
- Inkinen, J.; Mäkinen, R.; Keinänen-Toivola, M.M.; Nordström, K.; Ahonen, M. Copper as an antibacterial material in different facilities. Lett. Appl. Microbiol. 2017, 64, 19–26.
- Michels, H.T.; Keevil, C.W.; Salgado, C.D.; Schmidt, M.G. From laboratory research to a clinical trial: Copper alloy surfaces kill bacteria and reduce hospital-acquired infections. HERD: Health Environ. Resear. Design J. 2015, 9, 64–79.
- Wheeldon, L.J.; Worthington, T.; Lambert, P.A.; Hilton, A.C.; Lowden, C.J.; Elliott, T.S.J. Antimicrobial efficacy of copper surfaces against spores and vegetative cells of Clostridium difficile: The germination theory. J. Antimicrob. Chemother. 2008, 62, 522–525.
- Goudarzi, M.; Saviz, S.; Ghoranneviss, M.; Salar Elahi, A. Medical equipment antiseptic processes using the atmospheric plasma sprayed copper coatings. J. X-ray Sci. Technol. 2017, 25, 479–485.
- Silk, B.J.; Mahon, B.E.; Griffin, P.M.; Gould, L.H.; Tauxe, R.V.; Crim, S.M.; Jackson, K.A. Vital signs: Listeria illnesses, deaths, and outbreaks—United States, 2009–2011. MMWR. Morb. Mortal. Wkly. Rep. 2013, 62, 448.
- Zhu, Q.; Gooneratne, R.; Hussain, M.A. Listeria monocytogenes in fresh produce: Outbreaks, prevalence and contamination levels. Foods 2017, 6, 21.
- Wei, P.; Bao, R.; Fan, Y. Brainstem Encephalitis Caused by Listeria monocytogenes. Pathogens 2020, 9, 715.
- Aisha, A. Antimicrobial Effects of Copper and Brass Ions on the Growth of Listeria Monocytogenes at Different Temperatures, pH and Nutrients. Louisiana State University in Shreveport, USA, 2005. Available online: (accessed on 10 February 2021).
- Brennan, S.; McDonald, S.; McKenzie, J.; Cheng, A.; Green, S.; Allen, K. Systematic review of antimicrobial surfaces to reduce infection rates in hospitalized populations. Cochrane Aust. 2017, 1–21.
- Bauer, D.J. The chemotherapeutic activity of compounds of copper, rhodium and certain other metals in mice infected with neurovaccinia and ectromelia viruses. Br. J. Exp. Pathol. 1958, 39, 480. Available online: (accessed on 12 February 2021).
- Borkow, G.; Gabbay, J. Copper, an ancient remedy returning to fight microbial, fungal and viral infections. Curr. Chem. Biol. 2019, 3, 272–278.
- Fujimori, Y.; Sato, T.; Hayata, T.; Nagao, T.; Nakayama, M.; Nakayama, T.; Sugamata, R.; Suzuki, K. Novel antiviral characteristics of nanosized copper (I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl. Environ. Microbiol. 2012, 78, 951–955.
- Michels, H.T.; Anderson, D.G. Antimicrobial regulatory efficacy testing of solid copper alloy surfaces in the USA. In Met Ions Biology and Medicine; Collery, P., Marymard, I., Theophanides, T., Khassanova, L., Collery, T., Eds.; Copper Development Association Inc.: McLean, VA, USA, 2008; Volume 10, pp. 185–190. Available online: (accessed on 20 December 2020).
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. NEJM 2020, 382, 1564–1567.
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547.
- Warnes, S.L.; Keevil, C.W. Inactivation of norovirus on dry copper alloy surfaces. PLoS ONE 2013, 8, e75017.
- Science Daily. Copper Could Prevent the Spread of Flu Infections (14 February 2006). Available online: (accessed on 29 December 2020).
- ECI. Copper—A New Weapon to Fight the Influenza a Virus- New Research Finds Copper Effective at Inactivating H1N1 Virus. European Copper Institute. Available online: (accessed on 12 January 2021).
- Noyce, J.O.; Michels, H.; Keevil, C.W. Inactivation of influenza a virus on copper versus stainless steel surfaces. Appl. Environ. Microbiol. 2007, 73, 2748–2750.
- Warnes, S.L.; Little, Z.R.; Keevil, C.W. Human coronavirus 229E remains infectious on common touch surface materials. MBio 2015, 6, e01697-15.
- Cordis. Copper Could Stop Spread of Flu. Available online: (accessed on 12 January 2021).
- Santo, C.S.; Lam, E.W.; Elowsky, C.G.; Quaranta, D.; Domaille, D.W.; Chang, C.J.; Grass, G. Bacterial killing by dry metallic copper surfaces. Appl. Environ. Microbiol. 2011, 77, 794–802.
- Warnes, S.L.; Green, S.M.; Michels, H.T.; Keevil, C.W. Biocidal efficacy of copper alloys against pathogenic enterococci involves the degradation of genomic and plasmid DNAs. Appl. Environ. Microbiol. 2010, 76, 5390–5401.
- Michels, H.; Moran, W.; Michel, J. Antimicrobial properties of copper alloy surfaces, with a focus on hospital-acquired infections. Int. J. Met. 2008, 2, 47–56.
- Borkow, G.; Lara, H.H.; Covington, C.Y.; Nyamathi, A.; Gabbay, J. Deactivation of human immunodeficiency virus type 1 in medium by copper oxide-containing filters. Antimicrob. Agents Chemother. 2008, 52, 518–525.
- Minoshima, M.; Lu, Y.; Kimura, T.; Nakano, R.; Ishiguro, H.; Kubota, Y.; Hashimoto, K.; Sunada, K. Comparison of the antiviral effect of solid-state copper and silver compounds. J. Hazard Mater. 2016, 312, 1–7.
- Hang, X.; Peng, H.; Song, H.; Qi, Z.; Miao, X.; Xu, W. Antiviral activity of cuprous oxide nanoparticles against hepatitis C virus in vitro. J. Virol. Methods 2015, 222, 150–157.
- Chauhan, G.; Rath, G.; Goyal, A.K. In-vitroanti-viral screening and cytotoxicity evaluation of copper-curcumin complex. Artif. Cells Nanomed. Biotech. 2013, 41, 276–281.
- Shionoiri, N.; Sato, T.; Fujimori, Y.; Nakayama, T.; Nemoto, M.; Matsunaga, T.; Tanaka, T. Investigation of the antiviral properties of copper iodide nanoparticles against feline calicivirus. J. Biosci. Bioengin. 2012, 113, 580–586.
- ICA. The International Copper Association. Available online: (accessed on 11 February 2021).
- Sagripanti, J.L.; Routson, L.B.; Bonifacino, A.C.; Lytle, C.D. Mechanism of copper-mediated inactivation of herpes simplex virus. Antimicrob. Agents Chemother. 1997, 41, 812–817.
- Borkow, G.; Gabbay, J. Copper as a biocidal tool. Curr. Med. Chem. 2005, 12, 2163–2175.
- Cha, J.S.; Cooksey, D.A. Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc. Natl. Acad. Sci. USA 1991, 88, 8915–8919.
- Cooksey, D.A. copper uptake and resistance in bacteria. Mol. Microbiol. 1993, 7, 1–5.
- Kumbhar, A.S.; Padhye, S.B.; Saraf, A.P.; Mahajan, H.B.; Chopade, B.A.; West, D.X. Novel broad-spectrum metal-based antifungal agents. Biol. Met. 1991, 4, 141–143.
- Mumcuoglu, K.Y.; Gabbay, J.; Borkow, G. Copper oxide-impregnated fabrics for the control of house dust mites. Int. J. Pest Manag. 2008, 54, 235–240.
- Bellí, N.; Marín, S.; Sanchis, V.; Ramos, A.J. Impact of fungicides on Aspergillus carbonarius growth and ochratoxin A production on synthetic grape-like medium and on grapes. Food Addit. Contam. 2006, 23, 1021–1029.
- La Torre, A.; Iovino, V.; Caradonia, F. Copper in plant protection: Current situation and prospects. Phytopathol. Mediterr. 2018, 57, 201–236.
- Weaver, L.; Michels, H.T.; Keevil, C.W. Potential for preventing spread of fungi in air-conditioning systems constructed using copper instead of aluminium. Lett. Appl. Microbiol. 2010, 50, 18–23.
- Ballo, M.K.; Rtimi, S.; Kiwi, J.; Pulgarin, C.; Entenza, J.M.; Bizzini, A. Fungicidal activity of copper-sputtered flexible surfaces under dark and actinic light against azole-resistant Candida albicans and Candida glabrata. J. Photochem. Photobiol. B Biol. 2017, 174, 229–234.
- Creaven, B.S.; Duff, B.; Egan, D.A.; Kavanagh, K.; Rosair, G.; Thangella, V.R.; Waslh, M. Anticancer and antifungal activity of copper (II) complexes of quinolin-2 (1H)-one-derived Schiff bases. Inorg. Chim. Acta 2010, 363, 4048–4058.
- Ghasemian, E.; Naghoni, A.; Tabaraie, B.; Tabaraie, T. In vitro susceptibility of filamentous fungi to copper nanoparticles assessed by rapid XTT colorimetry and agar dilution method. J. Mycol. Médicale 2012, 22, 322–328.
- Usman, M.S.; El Zowalaty, M.E.; Shameli, K.; Zainuddin, N.; Salama, M.; Ibrahim, N.A. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomed. 2013, 8, 4467.
- Kruk, T.; Szczepanowicz, K.; Stefańska, J.; Socha, R.P.; Warszyński, P. Synthesis and antimicrobial activity of monodisperse copper nanoparticles. Colloids Surf. B Biointerfaces 2015, 128, 17–22.
- Mehtar, S.; Wiid, I.; Todorov, S.D. The antimicrobial activity of copper and copper alloys against nosocomial pathogens and Mycobacterium tuberculosis isolated from healthcare facilities in the Western Cape: An in-vitro study. J. Hosp. Infect. 2008, 68, 45–51.
- Quaranta, D.; Krans, T.; Santo, C.S.; Elowsky, C.G.; Domaille, D.W.; Chang, C.J.; Grass, G. Mechanisms of contact-mediated killing of yeast cells on dry metallic copper surfaces. Appl. Environ. Microbiol. 2011, 77, 416–426.
- Molteni, C.; Abicht, H.K.; Solioz, M. The killing of bacteria by copper surfaces involves dissolved copper. Appl. Environ. Microbiol. 2010, 76, 4099–4101.
- Santo, C.E.; Morais, P.V.; Grass, G. Isolation and characterization of bacteria resistant to metallic copper surfaces. Appl. Environ. Microbiol. 2010, 76, 1341–1348.
- Faúndez, G.; Troncoso, M.; Navarrete, P.; Figueroa, G. Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and Campylobacter jejuni. BMC Microbiol. 2004, 4, 1–7.
- Remyadevi, J.; Jeyasubramanian, K.; MArikani, A.; Rajkumar, G.; Rahuman, A.A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012, 71, 114–116.
- Balela, M.D.L.; Amores, K.L.S. Electroless deposition of copper nanoparticles for antimicrobial coating. Mater. Chem. Phys. 2019, 225, 393–398.
- Raffi, M.; Hussain, F.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Hasan, M.M. Investigations into the antibacterial behaviour of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80.
- Anita, S.; Ramachandran, T.; Rajendran, R.; Koushik, C.V.; Mahalakshmi, M.A. Study of the antimicrobial property of encapsulated copper oxide nanoparticles on cotton fabric. Text. Res. J. 2011, 81, 1081–1088.
- Harikumar, P.S.; Aravind, A. Antebacterial activity of copper nanoparticles and nanocomposites against Escherichia coli bacteria. Int. J. Sci. 2016, 5, 83–90.
- Niiyama, N.; Sasahara, T.; Mase, H.; Abe, M.; Saito, H.; Katsuoka, K. Use of copper alloy for preventing transmission of methicillin-resistant Staphylococcus aureus contamination in the dermatology ward. Acta Derm. Venereol. 2013, 93, 294–300.
- Palza, H.; Nuñez, M.; Bastías, R.; Delgado, K. In situ antimicrobial behavior of materials with copper-based additives in a hospital environment. Int. J. Antimicrob. Agents. 2018, 51, 912–917.
- Applerot, G.; Lellouche, J.; Lipovsky, A.; Nitzan, Y.; Lubart, R.; Gedanken, A.; Banin, E. Understanding the antibacterial mechanism of CuO nanoparticles: Revealing the route of induced oxidative stress. Small 2012, 8, 3326–3337.
- Giannousi, K.; Lafazanis, K.; Arvanitidis, J.; Pantazaki, A.; Dendrinou-Samara, C. Hydrothermal synthesis of copper-based nanoparticles: Antimicrobial screening and interaction with DNA. J. Inorg. Biochem. 2014, 133, 24–32.
- Dizaj, S.M.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial activity of carbon-based nanoparticles. Adv. Pharma. Bull. 2015, 5, 19.
- Padil, V.V.T.; Černík, M. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int. J. Nanomed. 2013, 8, 889.
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316.
- Griffitt, R.J.; Weil, R.; Hyndman, K.A.; Denslow, N.D.; Powers, K.; Taylor, D.; Barber, D.S. Exposure to copper nanoparti-cles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ. Sci. Technol. 2009, 41, 8178–8186.
- Chen, Z.; Meng, H.A.; Xing, G.M.; Chen, C.Y.; Zhao, Y.L.; Jia, G.A.; Wang, T.C.; Yuan, H.; Ye, C.; Zhao, F.; et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 163, 109–120.
- Edwards, M.; Sprague, N. Organic matter and copper corrosion by-product 455 release: A mechanistic study. Corros. Sci. 2001, 43, 1–18.
- Sabrià, M.; Garcia-Nunez, M.; Pedro-Botet, M.L.; Sopena, N.; Gimeno, J.M.; Reynaga, E.; Morera, J.; Rey-Joly, C. Presence and chromosomal subtyping of Legionella species in potable water systems in 20 hospitals of Catalonia, Spain. Infect. Control Hosp. Epidemiol. 2001, 22, 673–676.
- Cunha, B.A.; Burillo, A.; Bouza, E. Legionnaires’ disease. Lancet 2016, 387, 376–385.
- Sabria, M.; Victor, L.Y. Hospital-acquired legionellosis: Solutions for a preventable infection. Lancet Infect. Dis. 2002, 2, 368–373.
- Stout, J.E.; Victor, L.Y. Experiences of the first 16 hospitals using copper-silver ionization for Legionella control: Implications for the evaluation of other disinfection modalities. Infect. Control Hosp. Epidemiol. 2003, 24, 563–568.
- Cachafeiro, S.P.; Naveira, I.M.; García, I.G. Is copper–silver ionisation safe and effective in controlling legionella? J. Hosp. Infect. 2007, 67, 209–216.
- Casari, E.; Ferrario, A.; Montanelli, A. Prolonged effect of two combined methods for Legionella disinfection in a hospital water system. Ann. Ig. Med. Prev. Comunità 2007, 19, 525–532.
- Chen, Y.S.; Lin, Y.E.; Liu, Y.-C.; Hunag, W.K.; Shih, H.Y.; Wann, S.R.; Lee, S.S.; Tsai, H.C.; Li, C.H.; Chao, H.L.; et al. Efficacy of point-of-entry copper–silver ionisation system in eradicating Legionella pneumophila in a tropical tertiary care hospital: Implications for hospitals contaminated with Legionella in both hot and cold water. J. Hosp. Infect. 2008, 68, 152–158.
- Thneibat, A.; Cochiran, M.A.; Gonzalez-Cabezas, C.; Moore, B.K.; Matis, B.A.; Lund, M.R. Anticariogenic and antibacterial properties of a copper varnish using an in vitro microbial caries model. Oper. Dent. 2008, 33, 142–148.
- Mulligan, A.M.; Wilson, M.; Knowles, J.C. The effect of increasing copper content in phosphate-based glasses on biofilms of Streptococcus sanguis. Biomaterials 2003, 24, 1797–1807.
- Ditta, I.B.; Steele, A.; Liptrot, C.; Tobin, J.; Tyler, H.; Yates, H.M.; Sheel, D.W.; Foster, H.A. Photocatalytic antimicrobial activity of thin surface films of TiO2, CuO and TiO2/CuO dual layers on Escherichia coli and bacteriophage T4. Appl. Microbiol. Biotechnol. 2008, 79, 127.
- Santo, C.E.; Taudte, N.; Nies, D.H.; Grass, G. Contribution of copper ion resistance to survival of Escherichia coli on metallic copper surfaces. Appl. Environ. Microbiol. 2008, 74, 977–986.
- Abou Neel, E.A.; Ahmed, I.; Pratten, J.; Nazhat, S.N.; Knowles, J.C. Characterisation of antibacterial copper releasing degradable phosphate glass fibres. Biomaterials 2005, 26, 2247–2254.
- Guldiren, D.; Aydın, S. Antimicrobial property of silver, silver-zinc and silver-copper incorporated soda lime glass prepared by ion exchange. Mater. Sci. Eng. C 2017, 78, 826–832.
- Delgado, K.; Quijada, R.; Palma, R.; Palza, H. Polypropylene with embedded copper metal or copper oxide nanoparticles as a novel plastic antimicrobial agent. Lett. Appl. Microbiol. 2011, 53, 50–54.
- Sudha, V.P.; Ganesan, S.; Pazhani, G.P.; Ramamurthy, T.; Nair, G.B.; Venkatasubramanian, P. Storing drinking-water in copper pots kills contaminating diarrhoeagenic bacteria. J. Health Popul. Nutr. 2012, 30, 17.
- Sudha, V.B.P.; Singh, K.O.; Prasad, S.R.; Venkatasubramanian, P. Killing of enteric bacteria in drinking water by a copper device for use in the home: Laboratory evidence. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 819–822.
- Sharan, R.; Chhibber, S.; Attri, S.; Reed, R.H. Inactivation and sub-lethal injury of Escherichia coli in a copper water storage vessel: Effect of inorganic and organic constituents. Antonie Van Leeuwenhoek 2010, 98, 103–115.
- Borkow, G.; Gabbay, J. Putting copper into action: Copper- products with potent biocidal activities. FASEB J. 2004, 18, 1728–1730.
- Gabbay, J.; Borkow, G.; Mishal, J.; Magen, E.; Zatcoff, R.; Shemer-Avni, Y. Copper oxide impregnated textiles with potent biocidal activities. J. Ind. Text. 2006, 35, 323–335.
