AB5 compounds issued from the reactivity of hexachlorocyclotriphosphazene are relatively easy to obtain using two ways: either first the reaction of one chloride with one reagent, followed by the reaction of the five remaining Cl with another reagent, or first the reaction of five chlorides with one reagent, followed by the reaction of the single remaining Cl with another reagent. This particular property led to the use of such compounds as core for the synthesis of dendrons (dendritic wedges), using the five functions for growing the dendritic branches. The single function can be used for the synthesis of diverse types of dendrimers (onion peel, dumbbell-shape, Janus), for covalent or non-covalent grafting to solid surfaces, providing nanomaterials, for grafting a fluorophore, especially for studying biological mechanisms, or for self-associations to get micelles.
- cyclotriphosphazene
- dendron
- fluorescence
- phosphorus
- dendrimer
- nanomaterials
- sensors
- catalysis
- nanomedicine
1. Introduction
Hexachlorocyclotriphosphazene (N=PCl2)3 was synthesized a long time ago, in the first part of the 19th century [[1]], but correctly analyzed more than 30 years later [2]. Since that time, a huge number of reactions have been carried out, essentially of two types, either the thermal ring opening leading to polyphosphazenes [3], or nucleophilic substitutions of all the chlorides, most generally with alcohols/phenols or amines. An interesting feature of N3P3Cl6 is the possibility to differentiate the reactivity of one chlorine from the five others. Two different ways have been used to perform such differentiation: either one Cl reacts with one reagent, then the remaining five Cl react with another reagent, or 5 Cl react with one reagent, then the single remaining Cl reacts with another reagent (Scheme 1). The desired compounds are generally obtained in good yield after column chromatography.
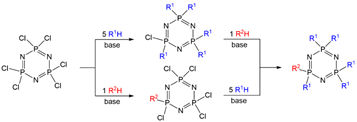
Scheme 1. Two ways for the synthesis of AB5 derivatives of cyclotriphosphazene.
However, the number of references concerning several types of AB5 (or A5B) derivatives of cyclotriphosphazene (searched on Scifinder®) demonstrates that the type of substitution reaction influences the preferred way. In addition, oxygen and nitrogen derivatives are widely used, whereas carbon derivatives are relatively rare. For reactions with amines or with carbon derivatives, it is largely preferred to replace first one chloride instead of five (83.5% for one N and 79.8% for one C) on N3P3Cl6. The situation is more contrasted when using oxygen derivatives (frequently phenols) as the percentage of publications displaying a single reaction is 61.4% .
The early experiences concerning the differentiation of the reactivity of the hexachlorocyclotriphosphazene have explored the possibility to selectively react one to six chlorides, in most cases using primary or, eventually, secondary amines. Such types of work were carried out, for instance, with dimethylamine [4], ter-butylamine [5], or isopropylamine [6]. The monosubstituted derivatives were isolated and characterized in all cases, as well as several di-, tri-, tetra-substituted derivatives, and the hexa-substituted derivatives, but not the penta-substituted ones. Other early examples of reactivity concerned enolate anions of acetaldehyde, acetone, and acetophenone, using lithium derivatives [7], methylsilane and methylsiloxane, using Grignard reagents [8], and phenols, using the sodium salt [9].
More recent results display a few practical uses of AB5 derivatives of cyclotriphosphazene, concerning in particular complexation properties, and polymerizations. For instance, the penta-phenoxy derivative was functionalized with a single 2-pyridylmethylamine ligand, then this compound was used for the complexation of Cu(NO3)2, PtCl2, and Co(NO3)2, affording a different type of complex in each case [10]. The reaction with FeX2 (X = ClO4− or BF4−) afforded complexes bearing two ligands around iron. These complexes are low spin below 300 K, but display spin crossover behavior above this temperature [11].
As indicated at the beginning of this introduction, cyclotriphosphazenes are widely used as precursors of polyphosphazenes. A few AB5 derivatives of cyclotriphosphazene have been used for such purpose. The Cl of the polyphosphazene were then replaced by reaction with sodium trifluoroethoxide, to avoid the hydrolytic instability of polydichlorophosphazenes [12][13][12,13]. A recent example of polymerization involved a substituent of the cyclotriphosphazene, thus it became a pendant group of the polymer[14].mer [14].
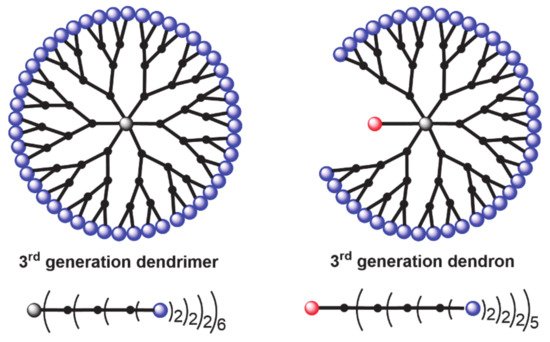
Being interested since a long time in the field of dendritic structures [15] and their applications [16], hexa-substituted cyclotriphosphazene have been widely used as core of dendrimers [17], but also in a few cases AB5 derivatives of cyclotriphosphazene have been used as building block for the construction of dendrimers. Another family of dendritic molecules is called “dendrons” when one function at the level of the core is different from the others, which are used for the growing of the branches. In the lower part of Figure 1 is represented another way to draw dendrimers and dendrons, i.e., a linear form, with parentheses after each layer of branching points, with a number indicating the multiplicity at the considered layer. Multiplication of all these numbers gives the number of terminal functions (2 × 2 × 2 × 6) = 48 for the dendrimer, 2 × 2 × 2 × 5 = 40 for the dendron, as shown in Figure 1.
The first part of this review will show AB5 derivatives of N3P3, including small dendrons, used for the synthesis of diverse types of dendrimers, including layered, dumbbell-shape and Janus dendrimers. The second part will concern the covalent or non-covalent grafting to diverse surfaces of dendrons based on a N3P3 core. The third part will concern the use in biology of fluorescent dendrons based on a N3P3 core. The fourth part will display other biological uses of dendrons based on a N3P3 core.
2. AB5 Derivatives of N3P3 as Core and Their Use for the Synthesis of Various Types of Dendrimers
A few examples of dendrimers having AB5 derivatives of N3P3 as branching points are known. In a first case, the synthesis consisted in reacting N3P3Cl6 used as core with a diamine. Then, the repetition of the first step was the reaction of the diamine with the five theoretically-remaining Cl on the cyclotriphosphazene [18]. However, the use at each step of reagents having two (diamine) or six (N3P3Cl6) identical functions precluded the obtaining of pure compounds.
For an accelerated synthesis of layered dendrimers having a precisely defined structure, two different types of AB5 derivatives of cyclotriphosphazene were synthesized, bearing five aldehydes and one azide or five phosphines and one hydrazine. Starting from a hexaaldehyde core (generation 0), the hydrazine derivative was condensed to afford a first-generation dendrimer functionalized with 30 phosphines. The third and last step of the synthesis process concerned again the condensation of the hydrazine derivative with the aldehyde terminal functions, to afford the third-generation dendrimer functionalized with 750 phosphines [19].
Another type of layered dendrimers was obtained by N-Boc-deprotection and subsequent N-chloroacetylation with chloroacetyl chloride and Hunig’s base, providing a chloride function as substituent of the core, which was further substituted by an azide group using NaN3. The azide was then reacted with monopropargylated tosyltetraethylene glycol under classical “click” reaction conditions, also named Huisgen (3 + 2) cycloaddition, between an azide and an alkyne in the presence of a copper catalyst, affording a triazole [20]. Then, the tosyl group was substituted with an azide group using NaN3. The resulting dendrimer had 90 allyl terminal groups, which were finally reacted with 1-thioglycerol, to afford a dendritic structure functionalized with 180 alcohol groups [21][22].
An extension of this work was carried out to get a series of dendritic molecules bearing lactoside terminal functions, starting in particular from a dendron bearing lactoside moieties on the surface and an azide at the core. Diverse examples of layered dendrimers were synthesized. The largest compound of this family of onion peel dendrimers contained up to 90 lactose moieties. The biological activity of these compounds was studied with a bacterial virulence factor, and human adhesion/growth-regulatory lectin, and showed multivalent effects [22][23].
Dendrimers built from two AB5 derivatives of N3P3, linked through a bifunctional fluorophore core, have dumbbell-shape structures. The synthesis was carried out using two repetitive steps [23], up[24], up to the second and third generations of dumbbell-shape dendrimers, and N,N-diethylethylenediamine was grafted as terminal groups in the last step, to induce solubility in water (Scheme 2). The dendrimer built from the fluorophore having alkene bonds (blue emitter) was used for imaging in vivo the vascular network of a rat olfactory bulb [24][25]. The dendrimer built from the fluorophore having alkyne bonds (green emitter) was used for imaging the blood vessels of a Xenopus laevis tadpole [25][26].
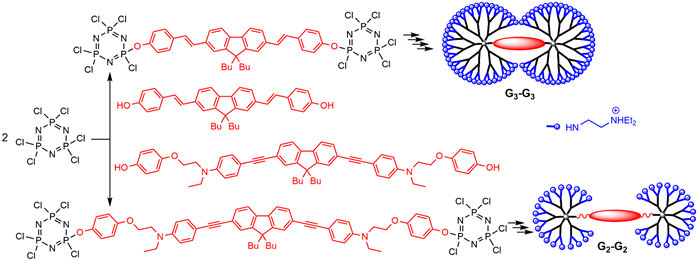
Scheme 2. Synthesis of “dumbbell-shape” dendrimers having two AB5 derivatives of N3P3 linked to a fluorescent core.
Two equivalents of the dendron bearing lactoside terminal functions were associated with a tetraethylene glycol core, functionalized with an alkyne on both sides. A double-click reaction in the presence of a copper catalyst provided another type of dumbbell-shape dendrimer [22][23].
The presence of a functional group at the core of dendrons can be used for grafting to a different dendron, to afford Janus dendrimers that are dendrimers possessing two different terminal functions, located in two areas (two faces) of the surface [26][27]. A series of Janus dendrimers was synthesized, in which one of the faces contained fluorescent groups, and the other face, water-solubilizing groups. Five equivalents of dansyl phenol were reacted to afford small fluorescent dendrons [27][28]. The second face of the Janus dendrimers was constituted by other dendrons having either a carboxylic acid or a hydrazide at the core and Boc-protected tyramine on the surface [28] (Sface [29] (Scheme 3).
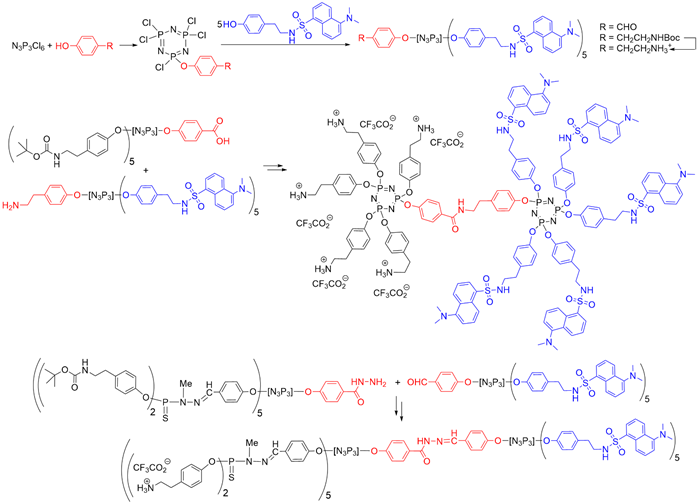
Scheme 3. Synthesis of fluorescent dendrons, and their association with other dendrons based on N3P3 for the synthesis of two types of Janus dendrimers.
Another example of Janus dendrimer was based on the dendron bearing lactoside terminal functions, which was associated by click reaction with another small dendron bearing a propargyl group at the core and five acetyl chloride functions. The presence of such functional groups on one side of this Janus dendrimer potentially offers the possibility to continue the growing of dendritic branches on one side [22][23].
3. Dendrons Based on AB5 Derivatives of N3P3 as Core and Their Use for the Synthesis of Nanomaterials
Dendrons based on cyclotriphosphazene as core were used for the covalent modification of different hard surfaces. Two small dendrons functionalized with five maleimide fluorophores, then with either two azabisphosphonate or two α-hydroxyphosphonate groups, were used for the grafting to thin films. The grafting was carried out on nanocrystalline mesoporous titania films, which became brightly fluorescent, using the first dendron. The titania film functionalized with the dendron having two azabisphosphonate groups was used as chemical sensor, which exhibited high sensitivity to phenolic OH moieties. A neat decrease in the fluorescence intensity of the films in the presence of phenol solutions was observed, especially with solutions containing either resorcinol or 2-nitroresorcinol [29].nol [30].
Several dendritic structures possessing two types of functions were elaborated both to be grafted to silica (triethoxysilane) and to be able to trap carbon dioxide (primary amines). Among these structures, a dendron based on the cyclotriphosphazene functionalized with one triethoxysilane and ten primary amines was synthesized [30][31]. This dendron was grafted to a porous silica of type SBA15 (pore diameter 6 nm). Then the Boc-protected amines were deprotected with trifluoroacetic acid (10%) in dichloromethane, followed by several washings. Measurements of CO2 trapping showed that this compound was active, but not the most active of the series, probably because it penetrated too far inside the pores of silica due to its small size [31][32].
Two different families of bifunctional water-soluble dendrons were synthesized. Both possess a single thioctic acid at the core, suitable for grafting to a thin gold layer coated on a glass surface. Generations 1 and 2 were synthesized and functionalized by either ammoniums or carboxylates as terminal functions, but only the first generations could be grafted to the gold layer. The surfaces modified by the positively or negatively charged dendrons were then used for studying their interaction with cells, in particular for the culture of human osteoblast cells (HOBC). It was shown that polycationic dendrons provoked cell apoptosis, whereas negatively charged dendrons supported cell adhesion and proliferation [32].[33].
A few examples concern the non-covalent (and thus reversible) π-stacking of dendrons functionalized with a pyrene at the core on graphene surfaces encapsulating cobalt nanoparticles (Co-NPs). At room temperature, in the presences of cobalt nanoparticles coated with a few graphene layers, the dendrons were associated to Co-NPs by π-stacking. When heating, the dendrons were dissociated from the Co-NPs, and performed catalysis in homogeneous conditions. Upon cooling, the dendrons associated back to the Co-NPs. Co nanoparticles are magnetic, thus the Co-NPs covered by the dendrons could be easily recovered using a magnet, and re-used. This concept was applied in particular to the synthesis of Felbinac, which is a nonsteroidal anti-inflammatory drug. The catalytic Co-NPs covered by the dendrons were recovered and reused 11 times, and afforded Felbinac with the same efficiency (quantitative yield) at the 12th run than at the first run (Scheme 4) [33].
[34].
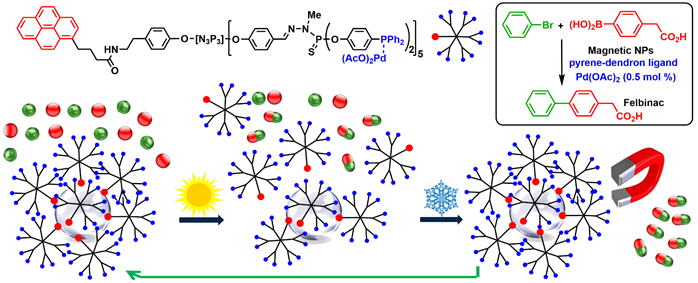
Scheme 4. Non-covalent grafting of catalytic dendrons onto cobalt nanoparticles covered by graphene layers, and their use as catalysts in Suzuki couplings. Example of coupling for the synthesis of Felbinac. Recovering and re-use were carried out eleven times, with a quantitative yield in Felbinac in all cases.
A small pyrene dendron equipped with the terpyridine ligand, suitable for the complexation of ruthenium complexes was non-covalently grafted as previously on the surface of cobalt NPs covered with graphene, and used in catalyzed nitroarene transfer hydrogenation from 2-propanol. Recycling and recovery of the catalysts-Co-NP with a magnet could be carried out seven times with still 100% yield in the eighth experiment with the dendritic catalyst [34][35].
Another pyrene-graphene association, was provided by a dendron functionalized with poly(vinylidene fluoride) (PVDF) polymers, using a Huisgen [3 + 2] cycloaddition (“click chemistry”). PVDF is an interesting fluoropolymer, constituted of CF2-CH2 units, which has remarkable properties [35][36]. The dendron interacting by π-stacking with the graphene surface of cobalt NPs covered by graphene layers displayed a thermo-responsive behavior [35].[37].
4. Fluorescent Dendrons Based on AB5 Derivatives of N3P3 as Core, Used in Biology
Diversely functionalized phosphorus dendrimers have many biological properties against cancers, chronic inflammations (rheumatoid arthritis, encephalomyelitis, psoriasis, etc.), tuberculosis, neurodegenerative diseases, and as carriers of biomolecules or drugs [36][38]. In order to decipher their mechanisms of action, it is often necessary to follow their behavior inside cells and in the body. To do that, a fluorescent group has been attached in many cases to the core of the dendrimer, thus providing a fluorescent dendron. The following paragraphs will be organized depending on the nature of the fluorophore linked to the cyclotriphosphazene core.
A single fluorescent maleimide fluorophore was linked at the core of a family of dendrons up to generation 2. The fluorescence quantum yield measured in dichloromethane decreased from 77 for the generation zero (5 aldehydes), to 22 for the first generation, and 20 for the second generation[37] [39]. The same family of dendrons was functionalized with ammonium terminal functions, instead of aldehydes, to afford water-soluble compounds (Figure 2). The fluorescence of the second generation was largely decreased in water compared to dichloromethane. This dendron was synthesized with the aim of monitoring transfection experiments, thanks to the presence of ammonium groups [38][40]. This dendron was able to interact with the plasmid DNA (BACE-GFP) [39][41].
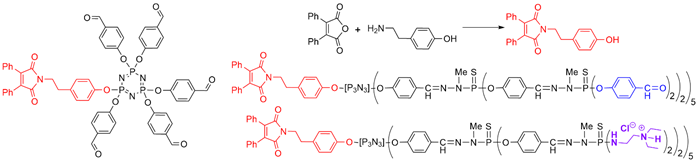
Figure 2. Dendrons functionalized with a single fluorescent maleimide at the core and with either 20 aldehyde or 20 ammonium terminal functions
The same family of dendrons, generations 1 and 2, was functionalized with cyclic ammoniums instead of linear ammoniums. Whatever the generation and the type of cyclic ammoniums, the dendrons built from the pyrene derivative at the core had a Critical Micelle Concentration (CMC) between 1 and 4 μM. The maleimide series less easily formed micelles, and displayed large differences in the CMC, depending on the generation of the dendron (Figure 3). These dendron-based micelles were tested against a panel of tumor cell lines[40] [42].
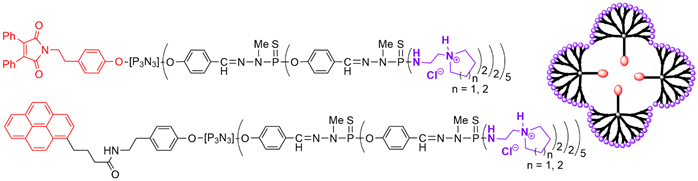
Figure 3. Amphiphilic dendrons functionalized with either maleimide or pyrene at the core and 20 cyclic ammoniums on the surface (2-(pyrrolidin-1-yl)ethan-1-amine (five-membered ring) or 2-(piperidin-1-yl)ethan-1-amine (six-membered ring). Schematized association of these dendrons in micelle.
A first-generation dendrimer having as terminal functions 12 azabisphosphonate groups based on tyramine was shown to be able to activate monocytes, which are a key cell population of the human innate immunity. In order to try to decipher the mechanisms and consequences of this activation, an analogous fluorescent dendron having the maleimide at the core and 10 azabisphosphonate terminal functions (Figure 4) was synthesized. The maleimide derivative was grafted in the very first step of the synthesis. It was used for FRET experiments (fluorescence resonance energy transfer) with phycoerythrin-coupled antibodies associated to different receptors, to determine the eventual involvement of a specific receptor [41][43].
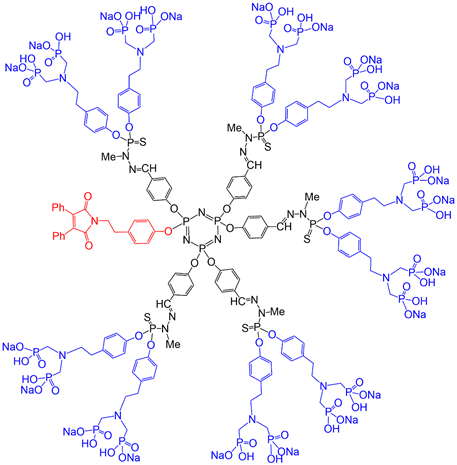
Figure 4. Full chemical structure of a first generation dendron having a maleimide at the core and 10 azabisphosphonate terminal functions
The same family of dendrimers/dendrons was shown to be able to multiply, frequently by several hundred folds, the number of natural killer (NK) cells, which are another key cell population of the human innate immunity, able to fight against viral and bacterial infections, and against many cancers. Indeed, it was demonstrated that this family of dendritic compounds offers an alternative strategy to the treatment of chronic inflammatory diseases such as rheumatoid arthritis [46] and multiple sclerosis [47]. Several generations of dendrimers bearing diverse negatively charged terminal functions were also synthesized, but the most active compound remained the first generation functionalized with azabisphosphonate functions [44]. It was shown later on that both the plain dendrimer (12 azabisphosphonate terminal functions) and the fluorescent dendron shown in Figure 4 (10 azabisphosphonate terminal functions) added to ex vivo cultures of peripheral blood mononuclear cells from healthy volunteers or from cancer patients with multiple myeloma enabled the efficient proliferation of NK cells, which were proved to exhibit in vivo anti-cancer activity [41][45].
In view of all the properties of the dendrimers ended by azabisphosphonate terminal functions, a structure/activity relationship was carried out, together with the desire to decipher their complex interactions with biological processes. Besides maleimide, other fluorescent groups were linked to the core of the dendrons, in particular based on julolidine to help in deciphering the biological mechanism of action. In this regard, having in hand several fluorescent analogs of a given compound with various λem/λex is somehow convenient to design biological experiments.
This fluorescent julolidine dendron was used to investigate the immunomodulatory effects of these dendritic azabisphosphonate derivatives. It was shown that they inhibited the activation, and therefore the proliferation, of CD4+T cells in cultures with IL-2 (interleukine) without affecting their viability. The presence of a larger quantity of IL-2 available induced the rapid enrichment of NK cells. This dendron was also efficient for inducing the activation of monocytes[42] [48]. The fluorescent dendron allowed to demonstrate a direct interaction with the T cells [43][49].
Dendrimers functionalized with other negatively charged groups, such as trans-cinnamic acid, were first used for the elaboration of nanostructured materials[44] [50]. The plain dendrimer and the corresponding fluorescent (julolidine) dendron having cinnamic acid terminal functions (Figure 5) were tested on membrane models (monolayers and multi-lamellar vesicles), and on monocytes. It was shown that the cinnamate dendrimer and dendron strongly disturb and alter the structure and the properties of model membranes, and they were found toxic towards monocytes. A totally different behavior was observed with the azabisphosphonate dendrimer and dendron, which had a low impact on the model membranes, and no toxicity towards monocytes [45][51].
The exact equivalent of the azabisphosphonate terminal functions was also synthesized in the carboxylate series, i.e., the azabiscarboxylate dendrimer (Figure 5) [46][52]. Interaction of these macromolecules with monocytes revealed that the fluorescent derivative having azabisphosphonate terminal functions can bind both non-specifically and specifically to the membrane of human monocytes. The specific binding led to the internalization of the phosphonate derivative by human monocytes. In contrast, the corresponding carboxylate derivative interacted only non-specifically with human monocytes, and was not internalized[47] [53].
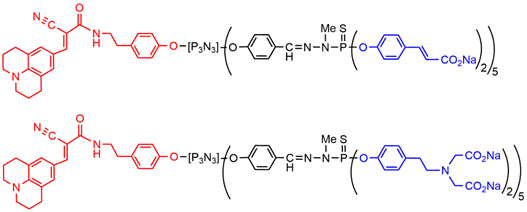
Figure 5. Examples of dendrons functionalized by one julolidine derivative at the core and 10 carboxylate or azabiscarboxylate terminal functions.
A large number of dendrimers functionalized in all cases with azabisphosphonate terminal functions were synthesized starting from different types of scaffolds, and tested for the activation of monocytes. Purely organic dendrimers (PAMAM, PPI and p-Lys) had no activity, whereas partly inorganic dendrimers, i.e., dendrimers bearing phosphorus or silicon at the branching points, were all active. The key feature was the “directionality” of the macromolecules, i.e., the repartition of the terminal functions as shown in the 3D modeling by molecular dynamics[48] [54]. All-atom molecular dynamics simulations of all these dendritic structures in explicit solvent demonstrated that the most directional compounds are the most active[49] [55].
On the way to clinical translation of this family of dendritic compounds, the biodistribution and the safety have to be assessed. The biodistribution in mice was first assessed with the julolidine dendron, then with the NIR dendron. The julolidine dendron injected in the mouse tail can be detected in draining lymph nodes and in the liver, and in very little proportion in the kidneys at day one. The julolidine derivative is brightly fluorescent in the green, but the autofluorescence of the tissues induces a green background which precludes a good quantification of the presence of the dendron, that is why the dendron functionalized with the NIR fluorophore was then used (Figure 6). It was mainly found in the lungs and in the liver, whereas lower quantities were detected in the lung and in the kidney. Fluorescence could be detected up to 56 days post injection[50] [56].
With the aim of broadening the scope of the anti-inflammatory properties of the azabisphosphonate dendritic compounds, their therapeutic efficacy in a preclinical mouse model of psoriasis induced by imiquimod was assessed. A moderate therapeutic effect was observed. The NIR dendron (Figure 6) was used for determining the skin permeation capability [51][57].
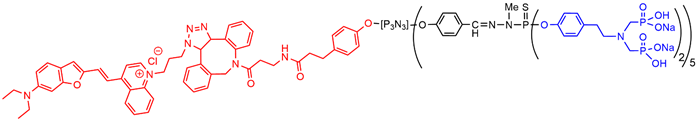
Figure 6. Dendron functionalized with a NIR fluorophore at the core and 10 azabisphosphonate derivatives on the surface.
4. Other Dendrons Based on AB5 Derivatives of N3P3 as Core, and Used in Biology
The family of dendritic structures having azabisphosphonate terminal functions has been expanded by decreasing the number of terminal active functions, in order to determine the influence of surface loading on the biological properties. The case of five reactive functions and one non-active has been exemplified in two previous Figures with a fluorophore being the non-reactive function (Figure 4 and Figure 6). The number of active functions was decreased, in order to determine the influence of surface loading on the biological properties. The growing of the dendritic branches was carried out starting from the remaining 5 to 1 chlorides, up to obtaining azabisphosphonate terminal functions (Figure 7) [42][48].
Screening the bioactivity of all these dendritic structures (Figure 7) towards human monocytes demonstrated that the compound built from the cyclotriphosphazene first functionalized with one equivalent of 2,2′-dihydroxybiphenyl, thus having eight azabisphosphonate terminal functions was still very active [42][48]. Recently the same series of dendritic structures was screened for the multiplication of NK cells. It was shown that the activation of monocytes is necessary in the framework of a multistep cross-talk between monocytes and NK cells. Thus, compounds that are not able to activate monocytes are not able to multiply NK cells [45].
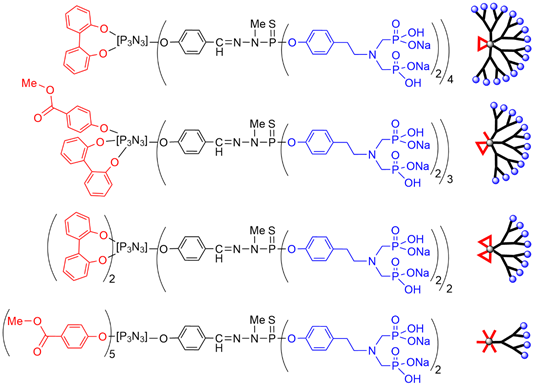
Figure 7. Chemical structure and schematization of dendritic structures having a decreased number of azabisphosphonate terminal functions.
Besides the fluorescent dendrons shown in Figure 3, dendrons functionalized with the same cyclic ammoniums on the surface and bearing one azabisphosphonate group at the core instead of the fluorophore were also synthesized. The azabisphosphonate groups are less hydrophobic than the fluorescent groups, thus the CMC values were higher, especially for the first generations (153.7 and 109 μM, for pyrrolidinium and piperidinium terminal groups, respectively), but also for the second generations (60 and 45.5 μM). As already observed with the fluorescent derivatives, dendrons containing piperidinium terminal groups displayed higher cytotoxicity than the corresponding dendrons containing pyrrolidinium terminal groups, and the second generation was more cytotoxic than the first. The second generation bearing the six-member ring ammonium was found to be much safer than free DOX when it treated A549, PC3, HCT116, and MDA-MB-231 tumor cell lines, as it displayed cell selectivity [40][42].
Dendrons having an alkyl chain (C11H23 or C17H35) at the core, and pyridine-imine functions on the surface, were found suitable for the complexation of metals such as copper or gold (Figure 8) [52][58]. The corresponding plain dendrimers were synthesized before, and complexed with either copper or gold, or with a mixture of both metals[53], [59], and an original mechanism of action was proposed [54][60]. Later on, it was shown that replacing copper by gold, i.e., CuCl2 by [AuCl2][AuCl4] strongly increased the antiproliferative activities against both KB and HL-60 tumoral cell lines, showing IC50s in the low nanomolar range [55][61]. Furthermore, the replacement of copper by gold considerably increased the anti-proliferative activity [52][58], as was shown previously with the corresponding third generation dendrimers.
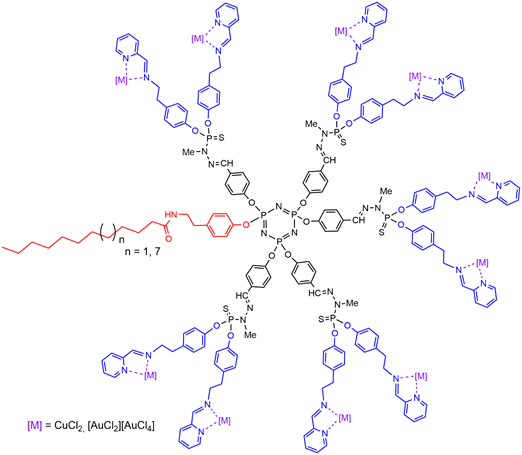
Figure 8. Dendrons having an alkyl chain at the core, and pyridine-imine functions complexing copper or gold on the surface.
In conclusion, the numerous properties of the AB5 derivatives of cyclotriphosphazene for the synthesis of diverse dendritic structures, such as layered dendrimers, dumbbell shape dendrimers, or Janus dendrimers has been shown in this review. Dendrons based on AB5 derivatives of cyclotriphosphazene have been already used in very different fields ranging from nanomaterials to catalysis or sensors, and to different fields of biology, in particular, to fight against chronic or acute inflammations and against different types of cancers. It should be emphasized that most properties were observed with small dendritic structures of generations 0 or 1, to avoid the burying, and thus the inefficiency of the single function at the core. Despite the numerous works already carried out, there is still plenty of room for the synthesis of other dendritic structures based on AB5 derivatives of cyclotriphosphazene. Furthermore, a few examples concerning A2B4 derivatives of cyclotriphosphazene in which two functions at the core are modified, while preserving most of the properties of dendritic structures have been published. One can also envisage the synthesis of ABC4 derivatives, having two different functions at the level of the core, potentially interesting in the fields of biology and nanomedicine, to incorporate specifically both a fluorophore and a targeting function, or whatever can be imagined.
References
- Liebig, J. Nachtrag der redaction. Ann. Pharm. 1834, 11–12, 139–150. [Google Scholar]Liebig, J.; Nachtrag der redaction. Ann. Pharm. 1834, 11–12, 139–150.
- Gladstone, J.H.; Holmes, J.D. On Chlorophosphuret of nitrogen, and its products of decomposition. J. Chem. Soc. 1864, 2, 225–237. [Google Scholar] [CrossRef]
- De Jaeger, R.; Gleria, M. Poly(organophosphazene)s and related compounds: Synthesis, properties and applications. Prog. Polym. Sci. 1998, 23, 179–276. [Google Scholar] [CrossRef]
- Keat, R.; Shaw, R.A. Phosphorus-Nitrogen compounds. Part IX. The reaction of dimethylamine with hexachlorocyclotriphosphazatriene: The replacement pattern and the structure of the products. J. Chem. Soc. 1965, 2215–2223. [Google Scholar] [CrossRef]
- Das, S.K.; Keat, R.; Shaw, R.A.; Smith, B.C. Phosphorus-Nitrogen compounds. Part XVI. The reactions of hexachlorocyclotriphosphazatriene with t-butylamine. J. Chem. Soc. 1965, 5032–5036. [Google Scholar] [CrossRef]
- Das, S.K.; Keat, R.; Shaw, R.A.; Smith, B.C. Phosphorus-Nitrogen compounds. Part XXII. The reactions of hexachlorocyclotriphosphazatriene with isopropylamine. J. Chem. Soc. A 1966, 1677–1680. [Google Scholar] [CrossRef]
- Harris, P.J.; Schwalke, M.A.; Liu, V.; Fisher, B.L. Phosphazenes.2. Synthesis of ketone-substituted and enol-substituted cyclotriphosphazenes. Inorg. Chem. 1983, 22, 1812–1817. [Google Scholar] [CrossRef]
- Allcock, H.R.; Brennan, D.J.; Graaskamp, J.M.; Parvez, M. Synthesis and molecular structure of methylsilane cyclotriphosphazenes and methylsiloxane cyclotriphosphazenes. Organometallics 1986, 5, 2434–2446. [Google Scholar] [CrossRef]
- Allcock, H.R.; Nelson, C.J.; Coggio, W.D. Synthesis and reactivity of cyclotriphosphazene bearing reactive silane functionalities: Novel derivatives via hydrosilylation reactions. Organometallics 1991, 10, 3819–3825. [Google Scholar] [CrossRef]
- Diefenbach, U.; Kretschmann, M.; Stromburg, B. Phosphazenes with (2-pyridylmethylamino) groups.1. Syntheses and crystal structures of pentaphenoxy(2-pyridylmethylamino)cyclotriphosphazene and its copper(II) nitrate, platinum(II) chloride, and cobalt(II) nitrate complexes. Chem. Ber. 1996, 129, 1573–1578. [Google Scholar] [CrossRef]
- Davidson, R.J.; Ainscough, E.W.; Brodie, A.M.; Jameson, G.B.; Waterland, M.R.; Moubaraki, B.; Murray, K.S.; Gordon, K.C.; Horvath, R.; Jameson, G.N.L. An iron(II) spin crossover grafted cyclotriphosphazene. Polyhedron 2013, 55, 37–44. [Google Scholar] [CrossRef]
- Scopelianos, A.G.; Obrien, J.P.; Allcock, H.R. Cyclic and polymeric phosphazenes with carborane side groups X-ray crystal structure of a carborane-substituted cyclophosphazene. J. Chem. Soc. Chem. Commun. 1980, 198–199. [Google Scholar] [CrossRef]
- Allcock, H.R.; Scopelianos, A.G.; Obrien, J.P.; Bernheim, M.Y. Synthesis and structure of carborane-substituted cyclic and polymeric phosphazenes. J. Am. Chem. Soc. 1981, 103, 350–357. [Google Scholar] [CrossRef]
- Kato, F.; Chandra, A.; Tokita, M.; Asano, H.; Shimomoto, H.; Ihara, E.; Hayakawa, T. Self-assembly of hierarchical structures using cyclotriphosphazene-containing poly(substituted methylene) block copolymers. ACS Macro Lett. 2018, 7, 37–41. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Turrin, C.-O.; Laurent, R.; Ouali, A.; Delavaux-Nicot, B. (Eds.) Dendrimers: Towards Catalytic, Material and Biomedical Uses; John Wiley & Sons Ltd.: Chichester, UK, 2011; pp. 1–538. [Google Scholar] [CrossRef]
- Caminade, A.M.; Ouali, A.; Laurent, R.; Turrin, C.O.; Majoral, J.P. Coordination chemistry with phosphorus dendrimers. Applications as catalysts, for materials, and in biology. Coord. Chem. Rev. 2016, 308, 478–497. [Google Scholar] [CrossRef]
- Slany, M.; Bardaji, M.; Casanove, M.J.; Caminade, A.M.; Majoral, J.P.; Chaudret, B. Dendrimer surface-chemistry—Facile route to polyphosphines and their gold complexes. J. Am. Chem. Soc. 1995, 117, 9764–9765. [Google Scholar] [CrossRef]
- Sournies, F.; Crasnier, F.; Graffeuil, M.; Faucher, J.P.; Lahana, R.; Labarre, M.C.; Labarre, J.F. Spherical Cyclophosphazene Dendrimers to the 5th Generation. Angew. Chem. Int. Ed. 1995, 34, 578–581. [Google Scholar] [CrossRef]
- Maraval, V.; Caminade, A.M.; Majoral, J.P.; Blais, J.C. Dendrimer design: How to circumvent the dilemma of a reduction of steps or an increase of function multiplicity? Angew. Chem. Int. Ed. 2003, 42, 1822–1826. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Sharma, R.; Zhang, I.; Abbassi, L.; Rej, R.; Maysinger, D.; Roy, R. A fast track strategy toward highly functionalized dendrimers with different structural layers: An “onion peel approach”. Polym. Chem. 2015, 6, 1436–1444. [Google Scholar] [CrossRef]
- Abbassi, L.; Chabre, Y.M.; Kottari, N.; Arnold, A.A.; Andre, S.; Josserand, J.; Gabius, H.J.; Roy, R. Multifaceted glycodendrimers with programmable bioactivity through convergent, divergent, and accelerated approaches using polyfunctional cyclotriphosphazenes. Polym. Chem. 2015, 6, 7666–7683. [Google Scholar] [CrossRef]
- Abbassi, L.; Chabre, Y.M.; Kottari, N.; Arnold, A.A.; Andre, S.; Josserand, J.; Gabius, H.J.; Roy, R.; Multifaceted glycodendrimers with programmable bioactivity through convergent, divergent, and accelerated approaches using polyfunctional cyclotriphosphazenes.. Polym. Chem. 2015, 6, 7666–7683.
- Mongin, O.; Rouxel, C.; Robin, A.C.; Pla-Quintana, A.; Krishna, T.R.; Recher, G.; Tiaho, F.; Caminade, A.M.; Majoral, J.P.; Blanchard-Desce, M. Brilliant organic nanodots: Novel nano-objects for bionanophotonics. In Nanobiosystems: Processing, Characterization, and Applications; Heckman, E.M., Singh, T.B., Yoshida, J., Eds.; Spie-Int Soc Optical Engineering: Bellingham, WA, USA, 2008; Volume 7040, p. 704006. [Google Scholar] [CrossRef]
- Caminade, A.M.; Laurent, R.; Delavaux-Nicot, B.; Majoral, J.P. “Janus” dendrimers: Syntheses and properties. New J. Chem. 2012, 36, 217–226. [Google Scholar] [CrossRef]
- Hameau, A.; Fuchs, S.; Laurent, R.; Majoral, J.P.; Caminade, A.M. Synthesis of dye/fluorescent functionalized dendrons based on cyclotriphosphazene. Beilstein J. Org. Chem. 2011, 7, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Pla-Quintana, A.; Mazeres, S.; Caminade, A.M.; Majoral, J.P. Cationic and Fluorescent “Janus” Dendrimers. Org. Lett. 2008, 10, 4751–4754. [Google Scholar] [CrossRef]
- Martinez-Ferrero, E.; Franc, G.; Mazeres, S.; Turrin, C.O.; Boissiere, U.; Caminade, A.M.; Majoral, J.P.; Sanchez, C. Optical properties of hybrid dendritic-mesoporous titania nanocomposite films. Chem. A Eur. J. 2008, 14, 7658–7669. [Google Scholar] [CrossRef]
- Riegert, D.; Pla-Quintana, A.; Fuchs, S.; Laurent, R.; Turrin, C.O.; Duhayon, C.; Majoral, J.P.; Chaumonnot, A.; Caminade, A.M. Diversified Strategies for the Synthesis of Bifunctional Dendrimeric Structures. Eur. J. Org. Chem. 2013, 5414–5422. [Google Scholar] [CrossRef]
- Riegert, D.; Bareille, L.; Laurent, R.; Majoral, J.P.; Caminade, A.M.; Chaumonnot, A. Silica Functionalized by Bifunctional Dendrimers: Hybrid Nanomaterials for Trapping CO2. Eur. J. Inorg. Chem. 2016, 3103–3110. [Google Scholar] [CrossRef]
- de Jong, E.R.; Deloch, N.; Knoll, W.; Turrin, C.O.; Majoral, J.P.; Caminade, A.M.; Koper, I. Synthesis and characterization of bifunctional dendrimers: Preliminary use for the coating of gold surfaces and the proliferation of human osteoblasts (HOB). New J. Chem. 2015, 39, 7194–7205. [Google Scholar] [CrossRef]
- Keller, M.; Colliere, V.; Reiser, O.; Caminade, A.M.; Majoral, J.P.; Ouali, A. Pyrene-Tagged Dendritic Catalysts Noncovalently Grafted onto Magnetic Co/C Nanoparticles: An Efficient and Recyclable System for Drug Synthesis. Angew. Chem. Int. Ed. 2013, 52, 3626–3629. [Google Scholar] [CrossRef]
- Asri, H.; Dautel, O.; Ouali, A. Terpyridine-Ru Complexes Noncovalently Supported on Cobalt Magnetic Nanoparticles for Nitroarene Transfer Hydrogenation. ACS Appl. Nano Mater. 2020, 3, 11811–11818. [Google Scholar] [CrossRef]
- Zapsas, G.; Patil, Y.; Gnanou, Y.; Ameduri, B.; Hadjichristidis, N. Poly(vinylidene fluoride)-based complex macromolecular architectures: From synthesis to properties and applications. Prog. Polym. Sci. 2020, 104, 101231. [Google Scholar] [CrossRef]
- Folgado, E.; Guerre, M.; Mimouni, N.; Colliere, V.; Bijani, C.; Ching, K.M.C.; Caminade, A.M.; Ladmiral, V.; Ameduri, B.; Ouali, A. pi-Stacking Interactions of Graphene-Coated Cobalt Magnetic Nanoparticles with Pyrene-Tagged Dendritic Poly(Vinylidene Fluoride). ChemPlusChem 2019, 84, 78–84. [Google Scholar] [CrossRef]
- Caminade, A.-M. Phosphorus dendrimers for nanomedicine. Chem. Commun. 2017, 53, 9830–9838. [Google Scholar] [CrossRef]
- Franc, G.; Mazeres, S.; Turrin, C.O.; Vendier, L.; Duhayon, C.; Caminade, A.M.; Majoral, J.P. Synthesis and properties of dendrimers possessing the same fluorophore(s) located either peripherally or off-center. J. Org. Chem. 2007, 72, 8707–8715. [Google Scholar] [CrossRef]
- Christophe Loup; Maria-Antonietta Zanta; Anne-Marie Caminade; Jean-Pierre Majoral; Bernard Meunier; Preparation of Water-Soluble Cationic Phosphorus-Containing Dendrimers as DNA Transfecting Agents. Chemistry – A European Journal 1999, 5, 3644-3650, 10.1002/(sici)1521-3765(19991203)5:12<3644::aid-chem3644>3.3.co;2-9.
- Kazmierczak-Baranska, J.; Pietkiewicz, A.; Janicka, M.; Wei, Y.Q.; Turrin, C.O.; Majoral, J.P.; Nawrot, B.; Caminade, A.M. Synthesis of a Fluorescent Cationic Phosphorus Dendrimer and Preliminary Biological Studies of Its Interaction with DNA. Nucleosides Nucleotides Nucleic Acids 2010, 29, 155–167. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, L.; Zhan, M.; Laurent, R.; Bignon, J.; Mignani, S.; Shi, X.; Caminade, A.-M.; Majoral, J.-P. Facile Synthesis of Amphiphilic Fluorescent Phosphorus Dendron-Based Micelles as Antiproliferative Agents: First Investigations. Bioconjug. Chem. 2021, 32, 339–349. [Google Scholar] [CrossRef]
- Poupot, M.; Turrin, C.O.; Caminade, A.M.; Fournie, J.J.; Attal, M.; Poupot, R.; Fruchon, S. Poly(phosphorhydrazone) dendrimers: Yin and yang of monocyte activation for human NK cell amplification applied to immunotherapy against multiple myeloma. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Rolland, O.; Griffe, L.; Poupot, M.; Maraval, A.; Ouali, A.; Coppel, Y.; Fournie, J.J.; Bacquet, G.; Turrin, C.O.; Caminade, A.M.; et al. Tailored control and optimisation of the number of phosphonic acid termini on phosphorus-containing dendrimers for the ex-vivo activation of human monocytes. Chem. A Eur. J. 2008, 14, 4836–4850. [Google Scholar] [CrossRef]
- Portevin, D.; Poupot, M.; Rolland, O.; Turrin, C.O.; Fournie, J.J.; Majoral, J.P.; Caminade, A.M.; Poupot, R. Regulatory activity of azabisphosphonate-capped dendrimers on human CD4(+) T cell proliferation enhances ex-vivo expansion of NK cells from PBMCs for immunotherapy. J. Transl. Med. 2009, 7, 13. [Google Scholar] [CrossRef]
- Soler-Illia, G.; Rozes, L.; Boggiano, M.K.; Sanchez, C.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P. New mesotextured hybrid materials made from assemblies of dendrimers and titanium(IV)-oxo-organo clusters. Angew. Chem. Int. Ed. 2000, 39, 4250–4254. [Google Scholar] [CrossRef]
- Ielasi, F.; Ledall, J.; Anes, A.P.; Fruchon, S.; Caminade, A.M.; Poupot, R.; Turrin, C.O.; Blanzat, M. Influence of PPH dendrimers’ surface functions on the activation of human monocytes: A study of their interactions with pure lipid model systems. Phys. Chem. Chem. Phys. 2016, 18, 21871–21880. [Google Scholar] [CrossRef]
- Rolland, O.; Turrin, C.O.; Bacquet, G.; Poupot, R.; Poupot, M.; Caminade, A.M.; Majoral, J.P. Efficient synthesis of phosphorus-containing dendrimers capped with isosteric functions of amino-bismethylene phosphonic acids. Tetrahedron Lett. 2009, 50, 2078–2082. [Google Scholar] [CrossRef]
- Ledall, J.; Fruchon, S.; Garzoni, M.; Pavan, G.M.; Caminade, A.M.; Turrin, C.O.; Blanzat, M.; Poupot, R. Interaction studies reveal specific recognition of an anti-inflammatory polyphosphorhydrazone dendrimer by human monocytes. Nanoscale 2015, 7, 17672–17684. [Google Scholar] [CrossRef]
- Caminade, A.M.; Fruchon, S.; Turrin, C.O.; Poupot, M.; Ouali, A.; Maraval, A.; Garzoni, M.; Maly, M.; Furer, V.; Kovalenko, V.; et al. The key role of the scaffold on the efficiency of dendrimer nanodrugs. Nature Commun. 2015, 6, 7722. [Google Scholar] [CrossRef]
- Hayder, M.; Garzoni, M.; Bochicchio, D.; Caminade, A.-M.; Couderc, F.; Ong-Meang, V.; Davignon, J.-L.; Turrin, C.-O.; Pavan, G.M.; Poupot, R. Three-Dimensional Directionality Is a Pivotal Structural Feature for the Bioactivity of Azabisphosphonate-Capped Poly(PhosphorHydrazone) Nanodrug Dendrimers. Biomacromolecules 2018, 19, 712–720. [Google Scholar] [CrossRef]
- Fruchon, S.; Bellard, E.; Beton, N.; Goursat, C.; Oukhrib, A.; Caminade, A.-M.; Blanzat, M.; Turrin, C.-O.; Golzio, M.; Poupot, R. Biodistribution and Biosafety of a Poly(Phosphorhydrazone) Dendrimer, an Anti-Inflammatory Drug-Candidate. Biomolecules 2019, 9, 475. [Google Scholar] [CrossRef]
- Jebbawi, R.; Oukhrib, A.; Clement, E.; Blanzat, M.; Turrin, C.-O.; Caminade, A.-M.; Lacoste, E.; Fruchon, S.; Poupot, R. An Anti-Inflammatory Poly(PhosphorHydrazone) Dendrimer Capped with AzaBisPhosphonate Groups to Treat Psoriasis. Biomolecules 2020, 10, 949. [Google Scholar] [CrossRef]
- Chen, L.; Fan, Y.; Qiu, J.; Laurent, R.; Li, J.; Bignon, J.; Mignani, S.; Caminade, A.-M.; Shi, X.; Majoral, J.-P. Potent Anticancer Efficacy of First-In-Class Cu-II and Au-III Metaled Phosphorus Dendrons with Distinct Cell Death Pathways. Chem. A Eur. J. 2020, 26, 5903–5910. [Google Scholar] [CrossRef]
- El Brahmi, N.; El Kazzouli, S.; Mignani, S.M.; Essassi, E.; Aubert, G.; Laurent, R.; Caminade, A.M.; Bousmina, M.M.; Cresteil, T.; Majoral, J.P. Original Multivalent Copper(II)-Conjugated Phosphorus Dendrimers and Corresponding Mononuclear Copper(II) Complexes with Antitumoral Activities. Mol. Pharm. 2013, 10, 1459–1464. [Google Scholar] [CrossRef]
- Mignani, S.; El Brahmi, N.; Eloy, L.; Poupon, J.; Nicolas, V.; Steinmetz, A.; El Kazzouli, S.; Bousmina, M.M.; Blanchard-Desce, M.; Caminade, A.M.; et al. Anticancer copper(II) phosphorus dendrimers are potent proapoptotic Bax activators. Eur. J. Med. Chem. 2017, 132, 142–156. [Google Scholar] [CrossRef]
- Mignani, S.M.; El Brahmi, N.; El Kazzouli, S.; Laurent, R.; Ladeira, S.; Caminade, A.-M.; Pedziwiatr-Werbicka, E.; Szewczyk, E.M.; Bryszewska, M.; Bousmina, M.M.; et al. Original Multivalent Gold(III) and Dual Gold(III)-Copper(II) Conjugated Phosphorus Dendrimers as Potent Antitumoral and Antimicrobial Agents. Mol. Pharm. 2017, 14, 4087–4097. [Google Scholar] [CrossRef]
