ŁEatwo pozyskiwany śluz z różnych części roślin jest bezwonną, bezbarwną i pozbawioną smakusily sourced mucus from various plant parts is an odorless, colorless and tasteless substancją o pojawiającym sięe with emerging commercial potencjale komercyjnym w rolnictwie, żywności, kosmetykach i ftial in agriculture, food, cosmetics and pharmaceutykach ze względu na swoje nietoksyczne iicals due to its non-toxic and biodegradowalne właściwościable properties. SItwierdzono, że śluz pochodzenia roślinnego może być stosowany jako has been found that plant-derived mucilage can be used as a naturalny zagęszczacz lub emulgator oraz thickener or emulsifier and an alternatywa dla syntetycznych polimerów i dodatkówive to synthetic polymers and additives. PoniBeważ jest niewidzialną barierą oddzielającą powierzchnię od otaczającejcause it is an invisible barrier that separates the surface from the surrounding atmosfery, jest stosowany jako jadalne powłoki przedłużające okres przydatności do spożycia świeżych warzyw i owoców oraz wielu produktów spożywczychphere, it is used as edible coatings to extend the shelf life of fresh vegetables and fruits as well as many food products. OpróczIn swoich właściwości funkcjonalnych, śluz może być również wykorzystywany doaddition to its functional properties, mucilage can also be used for the produkcji nanonośnikówction of nanocarriers. SkWe focupiamy się na metodach ekstrakcji śluzu i jego wykorzystaniu jakos on mucus extraction methods and its use as a naturalnego konserwantu dla świeżyc preservative for fresh produktówce. Wyszcze dególniliśmy kluczowe właściwości związane z ekstrakcją i konserwacją żywności,tailed the key properties related to the extraction and preservation of food, the mechanizm wpływu śluzu na właściwoścism of the effect of mucus on the sensoryczne properties of produktów, metody powlekania przy użyciu śluzu oraz jego recepturę na konserwowanie owoców i warzywcts, coating methods when using mucus and its recipe for preserving fruit and vegetables. ZUnderozumienie ekstanding the ecologicznych, ekal, economicznych i naukowych czynników and scientific factors of produkcji oraz efektywności śluzu jako wielokierunkowego środka otworzy jego praktyczne zastosowanie w wielu gałęziach przemysłuction and the efficiency of mucus as a multi-directional agent will open up its practical application in many industries.
- nanohydrogel
- food applications
- biopolymers
- polysaccharide
1. Wstęp1. Introduction
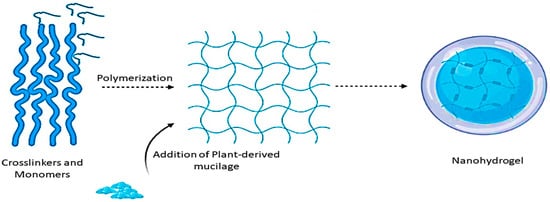
Polimery pochodzenia roślinnego cieszą się dużym zainteresowaniem w przemyśle spożywczym i innych ze względu na ich różnorodne zastosowania przemysłowe, takie jak powlekanie filmem, emulgator, spoiwo i środki żelujące, dlatego są nadmiernie wykorzystywane w przemyśle włókienniczym, papierniczym i kosmetycznym [ 1] , 2 ]. Obecnie, ze względu na niebezpieczny wpływ polimerów syntetycznych na zdrowie człowieka, ludzie wykazywali duże zainteresowanie biopolimerami pochodzenia roślinnego (guma, śluz, celuloza i glukany) jako skutecznym składnikiem do formułowania przyjaznych dla środowiska, zrównoważonych, kosztownych -skuteczne produkty [ 3 ]. Co więcej, duża liczba polisacharydów może być również wytwarzana biosyntetycznie przez kilka żywych organizmów, w tym rośliny, glony, zwierzęta, bakterie i grzyby [4 ]. Również naturalne polisacharydy są wykorzystywane w przemyśle spożywczym, ponieważ uważane są za bezpieczne do spożycia przez ludzi [ 5 ]. Spośród różnych polisacharydów śluz pochodzenia roślinnego jest szeroko stosowany w różnych gałęziach przemysłu spożywczego ze względu na jego cenne zastosowania o szerokim spektrum działania [ 6 ]. Zasadniczo można klej można otrzymać z różnych roślin lub ich poszczególnych części, takie jak aloe vera , Salvia hispanica nasion Cordia dichotoma , Basella alba, babki płesznika, Cyamopsis tetragonoloba , Cactaceae, Abelmoschus esculentus, Trigonella foenum-graecum , Moringa oleifera, i Linum usitatissimum .Śluz pochodzenia roślinnego, ze względu na swoje charakterystyczne właściwości zdrowotne (przeciwnowotworowe, hamowanie enzymu konwertującego angiotensynę rozciąga się na cukrzycę i stymulacja odporności) oraz właściwości spożywcze, jest szeroko stosowany jako składnik aktywny do formułowania produktów farmaceutycznych, funkcjonalnych i nutraceutycznych [ 7]. ]. Strukturalnie śluz (kompleks polimerycznych polisacharydów) składa się głównie z węglowodanów o silnie rozgałęzionych strukturach, które składają się z jednostek monomerycznych L-arabinozy, D-ksylozy, D-galaktozy, L-ramnozy i kwasu galakturonowego. Zawierają również glikoproteiny i różne składniki bioaktywne, takie jak garbniki, alkaloidy i steroidy [ 8 , 9 , 10]. Również śluz wytwarza nieskończoną liczbę monosacharydów podczas hydrolizy, w zależności od rodzaju produktów hydrolizy otrzymanych ze względu na charakter polisacharydu. Można go również dalej klasyfikować na cukry pentozowe (ksylan) i cukry heksozowe (celuloza i skrobia) i może być uważany za składniki gumopodobne ze względu na ich podobne właściwości fizjologiczne. Jednak zarówno śluz, jak i guma są głównie związane z hemicelulozami w składzie, z wyjątkiem cukrów wytwarzanych przez hemicelulozy, takich jak ksyloza, glukoza i mannoza, zamiast cukrów wytwarzanych przez gumy, takich jak galaktoza i arabinoza [ 11 , 12].]. Co więcej, można je wykorzystać w kilku zastosowaniach, takich jak powlekanie jadalne, gojenie ran, tworzenie tabletek, kapsułkowanie, oczyszczanie wody i różne nanonośniki. Śluzy wykazują doskonałe właściwości funkcjonalne, jednak ze względu na wiązania wodorowe pomiędzy różnymi grupami funkcyjnymi i innymi grupami polarnymi, odgrywają również ważną rolę w tworzeniu błony, emulsji, powlekanych nanocząstek metali i żelu [ 13 ]. W ostatnich latach nanostrukturalne hydrożele i nanocząstki metalowe pokryte śluzem są intensywnie wykorzystywane jako istotne nośniki dostarczania różnych składników hydrofilowych i hydrofobowych [ 14]]. Do formułowania nanohydrożelu można stosować różne rodzaje biopolimerów i polimerów sieciujących, a śluz może działać jako główny biopolimer lub składnik sieciujący do formułowania nanohydrożelu [ 15 ]. Opublikowano kilka raportów na temat formułowania stabilnych nanohydrożeli przy użyciu śluzu jako składnika aktywnego, a naukowcy ujawnili różne zastosowania terapeutyczne i spożywcze sformułowanych nanohydrożeli [ 11 , 12 , 13 , 14 , 15 , 16 , 17]. Ponadto nanohydrożele formułowane ze śluzem wykazują wyższą stabilność niż inne konwencjonalne biopolimery pochodzenia roślinnego. Ponadto nanocząsteczki metali pokryte polimerowymi węglowodanami, takimi jak skrobia, dekstran, chitozan i śluz, są najczęściej stosowanymi nanonośnikami do ukierunkowanego dostarczania leków. Ponieważ oprócz wydłużania czasu krążenia poprzez ukrywanie ich przed układem odpornościowym, ich polimerowe otoczki umożliwiają im przenoszenie i uwalnianie leku podczas biodegradacji [ 16 , 17 ].
2. Nanonośniki oparte na śluzie i ich zastosowanieMucilage Based Nanocarriers and Their Application
| Seed Mucilage | Nanocarrier | Applications | References | |||||
|---|---|---|---|---|---|---|---|---|
| Basil seed mucilage | Magnetic nanoparticles (Fe | 3 | O | 4 | ) | Application for the controlled delivery of antibiotic (Cephalexin) | [130] | [29] |
| Cress seed mucilage | Nanofibers | Application for the delivery of vitamin A | [18] | [30] | ||||
| Quince seed mucilage | Zinc oxide nanoparticles | Application for photocatalytic dye degradation | [131] | [31] | ||||
| Quince seed mucilage | Magnetic nanocomposites | Application for removal of cationic dyes from the aqueous solutions | [132] | [32] | ||||
| Basil seed mucilage | Zinc based magnetic bio nanocomposites | Application for removal of azo anionic and cationic dyes from the aqueous solutions | [133] | [33] | ||||
| Okra seed mucilage | Zinc oxide nanoparticles | Application for nanocomposites-based films | [91] | [26] | ||||
| Basil seed mucilage | ZnO nanocomposites | Application for wound healing | [68] | [34] | ||||
| Chia seed mucilage | Nanoencapsulation | Application as wall material | [115] | [35] |
References
- Ma, F.; Wang, R.; Li, X.; Kang, W.; Bell, A.E.; Zhao, D.; Liu, X.; Chen, W. Physical properties of mucilage polysaccharides from dioscorea Opposita Thunb. Food Chem. 2020, 311.
- Singh, R.; Barreca, D.N. Analysis of gums and mucilages. In Recent Advances in Natural Products Analysis; Silva, A.S., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 663–676.
- Freitas, T.K.F.S.; Oliveira, V.M.; de Souza, M.T.F.; Geraldino, H.C.L.; Almeida, V.C.; Fávaro, S.L.; Garcia, J.C. Optimization of coagulation-flocculation process for treatment of industrial textile wastewater using okra (A. esculentus) mucilage as natural coagulant. Ind. Crop. Prod. 2015, 76, 538–544.
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11.
- Chawla, P.; Kumar, N.; Bains, A.; Dhull, S.B.; Kumar, M.; Kaushik, R.; Punia, S. Gum arabic capped copper nanoparticles: Synthesis, characterization, and applications. Int. J. Biol. Macromol. 2020, 146, 232–242.
- Archana, G.; Sabina, K.; Babuskin, S.; Radhakrishnan, K.; Fayidh, M.A.; Azhagu Saravana Babu, P.; Sivarajan, M.; Sukumar, M. Preparation and characterization of mucilage polysaccharide for biomedical applications. Carbohydr. Polym. 2013, 98, 89–94.
- Ameri, A.; Heydarirad, G.; Jafari, J.M.; Ghobadi, A.; Rezaeizadeh, H.; Choopani, R. Medicinal plants contain mucilage used in traditional Persian medicine (TPM). Pharm. Biol. 2015, 53, 615–623.
- Fernandes, S.S.; de las Mercedes Salas-Mellado, M. Addition of chia seed mucilage for reduction of fat content in bread and cakes. Food Chem. 2017, 227, 237–244.
- Beikzadeh, S.; Khezerlou, A.; Jafari, S.M.; Pilevar, Z.; Mortazavian, A.M. Seed Mucilages as the functional ingredients for biodegradable films and edible coatings in the food industry. Adv. Colloid Interface Sci. 2020, 280, 102164.
- Gebresamuel, N.; Gebre-Mariam, T. Comparative physico-chemical characterization of the mucilages of two cactus pears (Opuntia Spp.) obtained from Mekelle, Northern Ethiopia. J. Biomater. Nanobiotechnol. 2012, 3, 79–86.
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P. Pharmaceutical applications of various natural gums, mucilages and their modified forms. Carbohydr. Polym. 2013, 92, 1685–1699.
- Petera, B.; Delattre, C.; Pierre, G.; Wadouachi, A.; Elboutachfaiti, R.; Engel, E.; Poughon, L.; Michaud, P.; Fenoradosoa, T.A. Characterization of arabinogalactan-rich mucilage from cereus triangularis cladodes. Carbohydr. Polym. 2015, 127, 372–380.
- Alpizar-Reyes, E.; Carrillo-Navas, H.; Gallardo-Rivera, R.; Varela-Guerrero, V.; Alvarez-Ramirez, J.; Pérez-Alonso, C. Functional properties and physicochemical characteristics of tamarind (Tamarindus indica L.) seed mucilage powder as a novel hydrocolloid. J. Food Eng. 2017, 209, 68–75.
- Hosseini, S.M.; Hemmati, K.; Ghaemy, M. Synthesis of nanohydrogels based on tragacanth gum biopolymer and investigation of swelling and drug delivery. Int. J. Biol. Macromol. 2016, 82, 806–815.
- Sharma, G.; Kumar, A.; Devi, K.; Sharma, S.; Naushad, M.; Ghfar, A.A.; Ahamad, T.; Stadler, F.J. Guar Gum-Crosslinked-Soya lecithin nanohydrogel sheets as effective adsorbent for the removal of thiophanate methyl fungicide. Int. J. Biol. Macromol. 2018, 114, 295–305.
- Oh, J.K.; Lee, D.I.; Park, J.M. Biopolymer-based microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2009, 34, 1261–1282.
- Suner, S.S.; Sahiner, M.; Sengel, S.B.; Rees, D.J.; Reed, W.F.; Sahiner, N. Responsive biopolymer-based microgels/nanogels for drug delivery applications. Stimuli Responsive Polym. Nanocarriers Drug Deliv. Appl. 2018, 1, 453–500.
- Sindhu, G.; Ratheesh, M.; Shyni, G.L.; Nambisan, B.; Helen, A. Anti-inflammatory and antioxidative effects of mucilage of Trigonella Foenum Graecum (fenugreek) on adjuvant induced arthritic rats. Int. Immunopharmacol. 2012, 12, 205–211.
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121.
- Sharma, G.; Naushad, M.; Kumar, A.; Rana, S.; Sharma, S.; Bhatnagar, A.; Stadler, F.J.; Ghfar, A.A.; Khan, M.R. Efficient removal of coomassie brilliant blue r-250 dye using starch/poly(alginic acid-cl-acrylamide) nanohydrogel. Process Saf. Environ. Prot. 2017, 109, 301–310.
- Lodhi, B.A.; Hussain, M.A.; Sher, M.; Haseeb, M.T.; Ashraf, M.U.; Hussain, S.Z.; Hussain, I.; Bukhari, S.N.A. Polysaccharide-based superporous, superabsorbent, and stimuli responsive hydrogel from sweet basil: A novel material for sustained drug release. Adv. Polym. Technol. 2019, 2119, 9583516.
- Setia, A.; Ahuja, P. Nanohydrogels. In Organic Materials as Smart Nanocarriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 293–368.
- Okutan, N.; Terzi, P.; Altay, F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocoll. 2014, 39, 19–26.
- Fathi, M.; Martín, Á.; McClements, D.J. Nanoencapsulation of food ingredients using carbohydrate based delivery systems. Trends Food Sci. Technol. 2014, 39, 18–39.
- Prusty, K.; Swain, S.K. Release of ciprofloxacin drugs by nano gold embedded cellulose grafted polyacrylamide hybrid nanocomposite hydrogels. Int. J. Biol. Macromol. 2019, 126, 765–775.
- Mohammadi, H.; Kamkar, A.; Misaghi, A. Nanocomposite films based on cmc, okra mucilage and ZnO nanoparticles: Physico mechanical and antibacterial properties. Carbohydr. Polym. 2018, 181, 351–357.
- Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent advances in natural gum-based biomaterials for tissue engineering and regenerative medicine: A review. Polymers 2020, 12, 176.
- Thakur, V.K.; Thakur, M.K. Recent Trends in Hydrogels Based on Psyllium Polysaccharide: A Review. J. Clean. Prod. 2014, 82, 1–15.
- Rayegan, A.; Allafchian, A.; Abdolhosseini Sarsari, I.; Kameli, P. Synthesis and characterization of basil seed mucilage coated Fe3O4 magnetic nanoparticles as a drug carrier for the controlled delivery of cephalexin. Int. J. Biol. Macromol. 2018, 113, 317–328.
- Mukherjee, T.; Lerma-Reyes, R.; Thompson, K.A.; Schrick, K. Making glue from seeds and gums: Working with plant-based polymers to introduce students to plant biochemistry. Biochem. Mol. Biol. Educ. 2019, 47, 468–475.
- Seyyed, M.; Tabrizi, H.M.; Behrouz, E.; Vahid, J. Biosynthesis of pure zinc oxide nanoparticles using Quince seed mucilage for photocatalytic dye degradation. J. Alloy. Compd. 2020, 821, 153519.
- Prasad, A.R.; Garvasis, J.; Oruvil, S.K.; Joseph, A. Improving oxidative and microbial stability of beef using Shahri Balangu seed mucilage loaded with Cumin essential oil as a bioactive edible coating. J. Phys. Chem. Solids 2019, 127, 265–274.
- Mahmoodi, M.; Javanbakht, V. Fabrication of Zn-based magnetic zeolitic imidazolate framework bionanocomposite using basil seed mucilage for removal of azo cationic and anionic dyes from aqueous solution. Int. J. Biol. Macromol. 2021, 167, 1076–1090.
- Kaur, M.; Kaur, R.; Punia, S. Characterization of mucilages extracted from different flaxseed (Linum usitatissiumum L.) cultivars: A heteropolysaccharide with desirable functional and rheological properties. Int. J. Biol. Macromol. 2018, 117, 919–927.
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419.
