Atopic dermatitis is one of the manifestations of atopic disease. In people, dermatitis is typically the first manifestation of atopic disease and can be followed by respiratory disease later in life as part of what is called the “atopic march”. Atopic dermatitis affects people and animals and, in some species (e.g., dogs), is the most prevalent manifestation of atopic disease. Atopic dermatitis in dogs has become increasingly common as exposure to indoor environments and processed foods has increased in our pets. Canine atopic dermatitis has characteristics, both clinically and immunologically, that are strikingly similar to the human counterpart. In dogs, progression to respiratory signs has been described in colonies of atopic dogs, but it does not seem to be a common observation in clinical practice.
1. Evolution of Our Understanding
Our comprehension of canine atopic dermatitis has greatly improved in the last decade [1]. The development of the clinical disease is the result of a complex interaction between genetic and environmental factors. While, in the past, IgE was considered the most important player in the pathogenesis, and much of the emphasis was placed on mast cells and histamine, it is now accepted that IgE may be an epiphenomenon. Many other factors besides IgE and histamine are now known to play a role in this complex [2]. Recent research has focused on the role of the skin barrier both in terms of ultrastructural alterations and dysbiosis [3] and the role of various lymphocytic populations [4][5][6]. This evolution in our understanding has been reflected in how clinical cases are managed. In the past, antihistamines were widely advocated for treatment and prevention of flares. Currently, the emphasis on the use of antihistamines has decreased [7], as controlled studies have failed to show the beneficial effect of antihistamines in a double-blind, placebo-controlled fashion [8]. Despite this, antihistamines may still be prescribed in practice [9] as part of a multimodal approach.
Our comprehension of canine atopic dermatitis has greatly improved in the last decade [9]. The development of the clinical disease is the result of a complex interaction between genetic and environmental factors. While, in the past, IgE was considered the most important player in the pathogenesis, and much of the emphasis was placed on mast cells and histamine, it is now accepted that IgE may be an epiphenomenon. Many other factors besides IgE and histamine are now known to play a role in this complex [10]. Recent research has focused on the role of the skin barrier both in terms of ultrastructural alterations and dysbiosis [11] and the role of various lymphocytic populations [12,13,14]. This evolution in our understanding has been reflected in how clinical cases are managed. In the past, antihistamines were widely advocated for treatment and prevention of flares. Currently, the emphasis on the use of antihistamines has decreased [15], as controlled studies have failed to show the beneficial effect of antihistamines in a double-blind, placebo-controlled fashion [16]. Despite this, antihistamines may still be prescribed in practice [17] as part of a multimodal approach.
As part of the newer approach, increased attention has been given to the restoration of the skin barrier through the application of sphingolipid and fatty acid emulsions [10][11][12]. This approach has been shown to restore some of the abnormalities of the atopic skin and have a positive effect on clinical signs. Topical therapy has also gained much emphasis for the treatment of secondary bacterial infections [13][14] due to the increase in antimicrobial resistance. Thus, while in the past, the use of topical antimicrobial products was considered an adjunctive therapy, now it is frequently advocated as a monotherapy whenever possible to minimize the use of systemic antibiotics.
As part of the newer approach, increased attention has been given to the restoration of the skin barrier through the application of sphingolipid and fatty acid emulsions [18,19,20]. This approach has been shown to restore some of the abnormalities of the atopic skin and have a positive effect on clinical signs. Topical therapy has also gained much emphasis for the treatment of secondary bacterial infections [21,22] due to the increase in antimicrobial resistance. Thus, while in the past, the use of topical antimicrobial products was considered an adjunctive therapy, now it is frequently advocated as a monotherapy whenever possible to minimize the use of systemic antibiotics.
2. Clinical Features, Allergy Tests, and How to Make A Diagnosis
Atopic dermatitis in dogs manifests as a pruritic inflammatory disease that affects body areas where the allergen is more readily absorbed epicutaneously. Examples of these areas are folds and areas with thinner skin and less hair. Examples include the antebrachial region, the axillae, and the inguinal area. The muzzle, periocular region, pinnae, and interdigital areas are other contact areas where exposure to the allergen is common. Thus, atopic dermatitis has characteristic predilected sites (
Figure 1
).
Figure 1.
The muzzle, periocular area, and ears are predilected sites for atopic dermatitis in dogs, as shown in this patient.
Each patient has some characteristic body areas that are prone to clinical flares. For example, some dogs present with inflammatory otitis externa, while others show their disease as pododermatitis. Recurrent otitis externa can be, for some patients, the only sign of atopic dermatitis for a while. Thus, it is important to control the underlying allergic process when patients develop secondary bacterial and yeast otitis on a seasonal basis.
It is important to realize, however, that the body sites affected by atopic dermatitis are not pathognomonic for this disease. For example, the face and feet can be affected by many other conditions, such as demodicosis or contact allergy. Thus, it is important to consider and rule out other differential diagnoses before making a clinical diagnosis of atopic dermatitis. We still lack a diagnostic test for atopic dermatitis. The diagnosis of atopic dermatitis is clinical and based on compatible history, clinical signs, and exclusion of other pruritic diseases. While we still rely on serology testing and intradermal testing to identify possible offending allergens to include in immunotherapy, it is understood that neither of these “tests” can be used for diagnostic purposes. Instead, “allergy tests” should be used
after a clinical diagnosis of atopic dermatitis has been made [15] with the main intent to formulate allergen-specific immunotherapy. This is an important concept in practice as these tests cannot be used to discriminate between an itchy dog due to atopic disease and an itchy dog affected by parasites. When considering serology tests, it is also important to note that the presence of IgEs against cross-reactive carbohydrate determinants has been documented in dogs. These IgEs are clinically irrelevant but can lead to many false positive results when using serology tests. Currently, some companies treat serum samples to block these IgEs to decrease false positive results and improve the accuracy of their serology test [16].
a clinical diagnosis of atopic dermatitis has been made [23] with the main intent to formulate allergen-specific immunotherapy. This is an important concept in practice as these tests cannot be used to discriminate between an itchy dog due to atopic disease and an itchy dog affected by parasites. When considering serology tests, it is also important to note that the presence of IgEs against cross-reactive carbohydrate determinants has been documented in dogs. These IgEs are clinically irrelevant but can lead to many false positive results when using serology tests. Currently, some companies treat serum samples to block these IgEs to decrease false positive results and improve the accuracy of their serology test [24].
3. Identification of Triggers
While foods can be trigger factors for atopic dermatitis in dogs, as is the case in children, we still tend to separate a food-induced disease from atopic dermatitis. Typically, the term atopic dermatitis is used to refer to a pruritic skin disease in which food and fleas have been ruled out, and we are left with a diagnosis by exclusion of “environmentally induced allergic dermatitis”. Thus, although foods are potential triggers of atopic dermatitis-type clinical signs, most clinicians still tend to use the term atopic dermatitis as equivalent to environmental allergies. The reality is that atopic dogs in which environmental triggers are important may be clinically indistinguishable to some in which foods are the trigger [17][18]. As avoidance of triggers is important in managing clinical flares, the identification of a possible role of foods in affecting the severity of the disease is a critical component of the management of nonseasonal atopic dogs. Importantly, there are some patients that look clinically indistinguishable from our classic atopic dogs but for whom environmental triggers cannot be identified, at least with the current tests available for use. For that subset of patients, we tend to use the terms “intrinsic atopic dermatitis or atopic-like disease” [19]. These cases represent an additional challenge as we are not able to use allergen-specific immunotherapy, and we are limited in only considering symptomatic therapies. Based on the current evidence in those cases, it appears that the clinical characteristics of those dogs are similar to the “extrinsic” cases in which an environmental trigger can be identified. The response to treatments also appears to be the same, although we only have very few studies with a low number of cases. This is an area in which we need to further examine this subpopulation to see if they constitute an early stage of the more classic atopic dermatitis or a specific subset. In other words, it is possible that these patients have not had sufficient time to develop an allergic response, and if they were tested later in life, they may be positive in our traditional tests. It may also be that these patients do not have skin barrier impairments, and that is a protective factor against the development of an epicutaneous sensitization toward environmental allergens.
While foods can be trigger factors for atopic dermatitis in dogs, as is the case in children, we still tend to separate a food-induced disease from atopic dermatitis. Typically, the term atopic dermatitis is used to refer to a pruritic skin disease in which food and fleas have been ruled out, and we are left with a diagnosis by exclusion of “environmentally induced allergic dermatitis”. Thus, although foods are potential triggers of atopic dermatitis-type clinical signs, most clinicians still tend to use the term atopic dermatitis as equivalent to environmental allergies. The reality is that atopic dogs in which environmental triggers are important may be clinically indistinguishable to some in which foods are the trigger [25,26]. As avoidance of triggers is important in managing clinical flares, the identification of a possible role of foods in affecting the severity of the disease is a critical component of the management of nonseasonal atopic dogs. Importantly, there are some patients that look clinically indistinguishable from our classic atopic dogs but for whom environmental triggers cannot be identified, at least with the current tests available for use. For that subset of patients, we tend to use the terms “intrinsic atopic dermatitis or atopic-like disease” [27]. These cases represent an additional challenge as we are not able to use allergen-specific immunotherapy, and we are limited in only considering symptomatic therapies. Based on the current evidence in those cases, it appears that the clinical characteristics of those dogs are similar to the “extrinsic” cases in which an environmental trigger can be identified. The response to treatments also appears to be the same, although we only have very few studies with a low number of cases. This is an area in which we need to further examine this subpopulation to see if they constitute an early stage of the more classic atopic dermatitis or a specific subset. In other words, it is possible that these patients have not had sufficient time to develop an allergic response, and if they were tested later in life, they may be positive in our traditional tests. It may also be that these patients do not have skin barrier impairments, and that is a protective factor against the development of an epicutaneous sensitization toward environmental allergens.
4. The Role of Skin Barrier in Canine Atopic Dermatitis
What we have learned in recent years is how important the skin barrier is for atopic dermatitis [20] and the propensity to epicutaneous sensitization to allergens [21]. Indeed, the epicutaneous route of exposure is important for both sensitization [22][23] and for elicitation of clinical signs [23][24]. While we are still lacking definitive evidence of a primary skin barrier impairment in atopic dogs, we do know that secondary skin barrier damage exists, as inflammation and self-trauma deteriorate the barrier function of the skin [25]. A damaged skin is more prone to absorb what it encounters and is prone to develop an allergic response to allergens. This is because damaged keratinocytes release cytokines such as thymic stromal lymphopoietin (TSLP), which promotes a T helper 2 response and the development of an allergic response. Thus, frequent removal of allergens from the skin of allergic dogs is crucial to minimize exposure and the worsening of inflammation. We know that the skin of atopic dogs is more alkaline and that it loses more water than normal skin. While in people, atopic skin is accepted to be drier than normal skin, the skin of atopic dogs does not appear to have decreased hydration, at least based on the current studies we have so far [26]. It is possible that our current methodologies are not sensitive enough or that despite the increased loss of water in atopic dogs, the hydration is still within normal limits. This could be linked to the fact that atopic dogs have been reported to have an increased gene expression of proteins such as filaggrin [27][28], whose breakdown products (natural moisturizing factors) are important for hydration.
What we have learned in recent years is how important the skin barrier is for atopic dermatitis [28] and the propensity to epicutaneous sensitization to allergens [29]. Indeed, the epicutaneous route of exposure is important for both sensitization [30,31] and for elicitation of clinical signs [31,32]. While we are still lacking definitive evidence of a primary skin barrier impairment in atopic dogs, we do know that secondary skin barrier damage exists, as inflammation and self-trauma deteriorate the barrier function of the skin [33]. A damaged skin is more prone to absorb what it encounters and is prone to develop an allergic response to allergens. This is because damaged keratinocytes release cytokines such as thymic stromal lymphopoietin (TSLP), which promotes a T helper 2 response and the development of an allergic response. Thus, frequent removal of allergens from the skin of allergic dogs is crucial to minimize exposure and the worsening of inflammation. We know that the skin of atopic dogs is more alkaline and that it loses more water than normal skin. While in people, atopic skin is accepted to be drier than normal skin, the skin of atopic dogs does not appear to have decreased hydration, at least based on the current studies we have so far [34]. It is possible that our current methodologies are not sensitive enough or that despite the increased loss of water in atopic dogs, the hydration is still within normal limits. This could be linked to the fact that atopic dogs have been reported to have an increased gene expression of proteins such as filaggrin [35,36], whose breakdown products (natural moisturizing factors) are important for hydration.
As the interest in the skin barrier and keratinization has increased in the past decade, several studies have investigated the role of filaggrin in canine atopic dermatitis. Filaggrin mutations have been reported as one of the most documented risk factors for atopic dermatitis in people [29][30]; thus, it was natural to address this issue in dogs. We now know that more than one filaggrin-type protein exists in dogs, although the exact function of filaggrin 2 in dogs is not clear [31]. Both filaggrin-type proteins are expressed in the upper layers of the skin and play a role in the differentiation of the epidermis and in the hydration of the skin. While in people, filaggrin loss-of-function mutations have been identified as major risk factors for the development of atopic dermatitis, this does not appear to be case in most breeds of dogs [32]. It is possible that in the future we will discover other players that may be more relevant for dogs. Research toward establishing a correlation between the severity of clinical signs and gene expression in the skin of atopic dogs has shown that genes relevant to skin barrier formation and immune function were altered [33]. Most of the studies on the genetics of canine atopic dermatitis had small sample sizes, which hindered their ability to detect factors given the heterogeneity of this condition and the variation of breeds [34].
As the interest in the skin barrier and keratinization has increased in the past decade, several studies have investigated the role of filaggrin in canine atopic dermatitis. Filaggrin mutations have been reported as one of the most documented risk factors for atopic dermatitis in people [37,38]; thus, it was natural to address this issue in dogs. We now know that more than one filaggrin-type protein exists in dogs, although the exact function of filaggrin 2 in dogs is not clear [39]. Both filaggrin-type proteins are expressed in the upper layers of the skin and play a role in the differentiation of the epidermis and in the hydration of the skin. While in people, filaggrin loss-of-function mutations have been identified as major risk factors for the development of atopic dermatitis, this does not appear to be case in most breeds of dogs [40]. It is possible that in the future we will discover other players that may be more relevant for dogs. Research toward establishing a correlation between the severity of clinical signs and gene expression in the skin of atopic dogs has shown that genes relevant to skin barrier formation and immune function were altered [41]. Most of the studies on the genetics of canine atopic dermatitis had small sample sizes, which hindered their ability to detect factors given the heterogeneity of this condition and the variation of breeds [42].
When challenged with an allergen, atopic dogs attempt to compensate for the insult with an increased production of filaggrin, and an increased expression of enzymes responsible for filaggrin degradation has been described in experimental models of canine atopic dermatitis [35]. The increased proteolytic activity of atopic skin has been reported in atopic people, and it is linked to the increased pH [36]. Clinically speaking, it may be important to acidify atopic skin to both decrease the proliferation of bacteria and yeasts and to decrease the proteolytic activity of ceramidases and proteases that degrade lipids in the skin and increase the rate of desquamation.
When challenged with an allergen, atopic dogs attempt to compensate for the insult with an increased production of filaggrin, and an increased expression of enzymes responsible for filaggrin degradation has been described in experimental models of canine atopic dermatitis [43]. The increased proteolytic activity of atopic skin has been reported in atopic people, and it is linked to the increased pH [44]. Clinically speaking, it may be important to acidify atopic skin to both decrease the proliferation of bacteria and yeasts and to decrease the proteolytic activity of ceramidases and proteases that degrade lipids in the skin and increase the rate of desquamation.
5. Factors Playing A Role in the Development of Clinical Disease
As mentioned previously, the actual development of clinical signs is the result of a combination of genetic and environmental factors [37]. Many genes have been considered as candidates for canine atopic dermatitis [38]. The increased prevalence of atopic dermatitis may not only be linked to a preferential breeding of atopic individuals but also to a change in environmental conditions. In people, according to the “hygiene theory”, it is documented that decreased exposure to parasites and beneficial bacteria predisposes one to the development of atopic dermatitis [39][40]. A similar situation may apply to our pets. While pets in the past were more exposed to an outdoor environment with less exposure to house dust mites and more exposure to parasites and bacteria, the current conditions of ingesting processed foods rather than raw diets, an increased exposure to indoor environments and house dust mites, and a decreased exposure to beneficial bacteria may contribute to an increased development of clinical signs of atopic dermatitis [41][42].
As mentioned previously, the actual development of clinical signs is the result of a combination of genetic and environmental factors [45]. Many genes have been considered as candidates for canine atopic dermatitis [46]. The increased prevalence of atopic dermatitis may not only be linked to a preferential breeding of atopic individuals but also to a change in environmental conditions. In people, according to the “hygiene theory”, it is documented that decreased exposure to parasites and beneficial bacteria predisposes one to the development of atopic dermatitis [47,48]. A similar situation may apply to our pets. While pets in the past were more exposed to an outdoor environment with less exposure to house dust mites and more exposure to parasites and bacteria, the current conditions of ingesting processed foods rather than raw diets, an increased exposure to indoor environments and house dust mites, and a decreased exposure to beneficial bacteria may contribute to an increased development of clinical signs of atopic dermatitis [49,50].
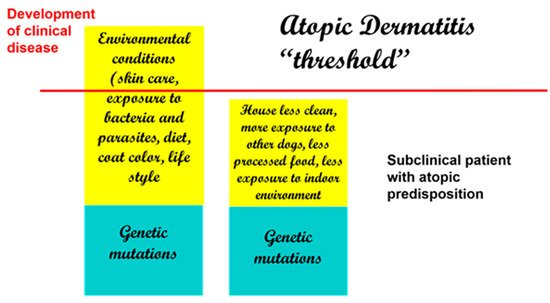
If there were an actual “threshold for development of atopic dermatitis” (
Figure 2
), we can assume that each factor is additive (from genetic factors to environmental factors) and that once the threshold is achieved, clinical disease ensues. Thus, when genetic factors are considered, it is possible that more than one may be necessary to lead to disease development.
Figure 2.
Development of clinical disease is the result of genetic and environmental factors. Decreased exposure to beneficial bacteria and increased exposure to indoor allergens and processed foods are some of the reported risk factors in dogs.
Therefore, with genetic factors being considered a constant, changes in environmental conditions can, by themselves, determine an increased development of clinical disease. This is supported by some studies in the veterinary literature that have linked the development of atopic dermatitis in dogs to dietary habits, lifestyle, and living conditions [43][44]. Of interest, coat color has been linked to the development of atopic dermatitis, and having more than 50% of a white coat color has been reported to be a risk factor [43].
Therefore, with genetic factors being considered a constant, changes in environmental conditions can, by themselves, determine an increased development of clinical disease. This is supported by some studies in the veterinary literature that have linked the development of atopic dermatitis in dogs to dietary habits, lifestyle, and living conditions [51,52]. Of interest, coat color has been linked to the development of atopic dermatitis, and having more than 50% of a white coat color has been reported to be a risk factor [51].
6. Phenotypes
The concept of phenotypes of atopic dermatitis has been investigated in human medicine for a long time. The term phenotype is intended to emphasize the interaction between genetics and environmental factors. Identification of phenotypes is important as we progress to a more personalized approach to treatment [45][46]. As atopic dermatitis is a heterogenous disease, identification of subgroups of patients is important for the sake of treatment success. Phenotypes of atopic dermatitis in people have been described based on age [47] and ethnicity [48][49][50]. In people, different subgroups appear to have peculiar pathways and key cytokines that play a role, thus requiring different treatments. In dogs, our knowledge of phenotypes is limited. Clinical phenotypes of canine atopic dermatitis have been described based on breeds and distribution of lesions, but no link has been drawn to specific markers and responses to treatment [51].
The concept of phenotypes of atopic dermatitis has been investigated in human medicine for a long time. The term phenotype is intended to emphasize the interaction between genetics and environmental factors. Identification of phenotypes is important as we progress to a more personalized approach to treatment [53,54]. As atopic dermatitis is a heterogenous disease, identification of subgroups of patients is important for the sake of treatment success. Phenotypes of atopic dermatitis in people have been described based on age [55] and ethnicity [56,57,58]. In people, different subgroups appear to have peculiar pathways and key cytokines that play a role, thus requiring different treatments. In dogs, our knowledge of phenotypes is limited. Clinical phenotypes of canine atopic dermatitis have been described based on breeds and distribution of lesions, but no link has been drawn to specific markers and responses to treatment [59].
7. Strategies for Treatment and Options Available for Atopic Dogs
New research has also focused on the identification of new targets for treatment to minimize the use of broad-spectrum therapies, such as glucocorticoids and cyclosporine, and to use more targeted approaches, such as biologics targeting key cytokines. In this respect, IL-31 has received much attention for its role in canine atopic dermatitis [52]. IL-31 is produced by TH2 cells, and many cells ranging from immune cells to keratinocytes and nerve fibers have receptors for this cytokine [53]. Its role in the mediation of pruritus has gained attention [54], but it important to emphasize that IL-31 also modulates keratinocyte proliferation and differentiation [55]. Strategies to target IL-31 in dogs have ranged from the use of a caninized monoclonal antibody [56] to vaccinating dogs against their own IL-31 [57], although this latter approach is currently only experimental. As atopic dermatitis is a syndrome with different pathomechanisms, not all dogs treated with a biologic aimed at targeting this cytokine respond. This is something to be expected as we move toward more targeted therapies. Nevertheless, this approach is revolutionary in veterinary medicine as biologics offer the freedom of not having to worry about drug interactions and can be considered for patients with a prior history of demodicosis or neoplasia, where other treatments may not be ideal.
New research has also focused on the identification of new targets for treatment to minimize the use of broad-spectrum therapies, such as glucocorticoids and cyclosporine, and to use more targeted approaches, such as biologics targeting key cytokines. In this respect, IL-31 has received much attention for its role in canine atopic dermatitis [60]. IL-31 is produced by TH2 cells, and many cells ranging from immune cells to keratinocytes and nerve fibers have receptors for this cytokine [61]. Its role in the mediation of pruritus has gained attention [62], but it important to emphasize that IL-31 also modulates keratinocyte proliferation and differentiation [63]. Strategies to target IL-31 in dogs have ranged from the use of a caninized monoclonal antibody [64] to vaccinating dogs against their own IL-31 [65], although this latter approach is currently only experimental. As atopic dermatitis is a syndrome with different pathomechanisms, not all dogs treated with a biologic aimed at targeting this cytokine respond. This is something to be expected as we move toward more targeted therapies. Nevertheless, this approach is revolutionary in veterinary medicine as biologics offer the freedom of not having to worry about drug interactions and can be considered for patients with a prior history of demodicosis or neoplasia, where other treatments may not be ideal.
Of major importance, we are now appreciating the value of a “proactive approach” when managing cases rather than a “reactive approach” [58]. As these dogs are very likely to flare at some point, it is important to do what is possible to prevent the flares rather than to wait for them to occur and then start treatment. This can be done with topical therapy in areas prone to flaring to minimize the need of rescue medications [59]. If we wait for the flares to occur, we may need more medication and of a larger spectrum, while the proactive approach can now be used with more targeted treatments such as lokivetmab [60]. Over time, fewer flares and fewer medications are needed to make the patient comfortable compared to the philosophy of waiting until the animal flares and then starting the treatment.
Of major importance, we are now appreciating the value of a “proactive approach” when managing cases rather than a “reactive approach” [66]. As these dogs are very likely to flare at some point, it is important to do what is possible to prevent the flares rather than to wait for them to occur and then start treatment. This can be done with topical therapy in areas prone to flaring to minimize the need of rescue medications [67]. If we wait for the flares to occur, we may need more medication and of a larger spectrum, while the proactive approach can now be used with more targeted treatments such as lokivetmab [68]. Over time, fewer flares and fewer medications are needed to make the patient comfortable compared to the philosophy of waiting until the animal flares and then starting the treatment.
Allergen-specific immunotherapy is still the only approach that may potentially alter the course of the disease and minimize future sensitizations. Different routes of administration have been reported in the literature, with the subcutaneous and the sublingual being the most commonly used in practice [61][62][63]. A recently published study directly compared the efficacy of subcutaneous with sublingual and intralymphatic and concluded that subcutaneous and intralymphatic were the most effective routes to improve clinical signs [64]. Allergen-specific immunotherapy is complementary to other treatments, as the efficacy takes time to manifest.
Allergen-specific immunotherapy is still the only approach that may potentially alter the course of the disease and minimize future sensitizations. Different routes of administration have been reported in the literature, with the subcutaneous and the sublingual being the most commonly used in practice [69,70,71]. A recently published study directly compared the efficacy of subcutaneous with sublingual and intralymphatic and concluded that subcutaneous and intralymphatic were the most effective routes to improve clinical signs [72]. Allergen-specific immunotherapy is complementary to other treatments, as the efficacy takes time to manifest.
Much attention has also been devoted to the identification of biomarkers [65], although it is not clear at this time if these proposed biomarkers are associated with specific responses to treatment. Cytokines such as TSLP [66], thymus and activation-regulated chemokine (TARC) [67], IL-33 [68], and IL-34 [69] have all been described in recent studies as possible biomarkers, and more studies are necessary to understand how these are relevant to a large population of atopic dogs. As we move toward a more targeted approach for the treatment of canine atopic dermatitis, it is reasonable to believe that more biologics targeting these cytokines will become available for dogs. It is important to emphasize that many of these cytokines are produced by keratinocytes (e.g., TSLP, IL-33) and that keratinocytes have the ability to shape the lymphocytic response toward allergens. Cytokines such as IL-33 and TSLP can promote an allergic/inflammatory response rather than tolerance. Additionally, some mediators released by keratinocytes such as TSLP and periostin [70] are able to directly elicit itch by acting on sensory nerves [71]. This is particularly relevant in chronic disease [72] in which increased density of nerve fibers [73] and enhanced peripheral sensitization play a role and can contribute to a decreased response to antipruritic therapy. Thus, keratinocytes are far from being a physical inert barrier, and they are an integral part of a cross talk with the nervous system and the immune cells.
Much attention has also been devoted to the identification of biomarkers [73], although it is not clear at this time if these proposed biomarkers are associated with specific responses to treatment. Cytokines such as TSLP [74], thymus and activation-regulated chemokine (TARC) [75], IL-33 [76], and IL-34 [77] have all been described in recent studies as possible biomarkers, and more studies are necessary to understand how these are relevant to a large population of atopic dogs. As we move toward a more targeted approach for the treatment of canine atopic dermatitis, it is reasonable to believe that more biologics targeting these cytokines will become available for dogs. It is important to emphasize that many of these cytokines are produced by keratinocytes (e.g., TSLP, IL-33) and that keratinocytes have the ability to shape the lymphocytic response toward allergens. Cytokines such as IL-33 and TSLP can promote an allergic/inflammatory response rather than tolerance. Additionally, some mediators released by keratinocytes such as TSLP and periostin [78] are able to directly elicit itch by acting on sensory nerves [79]. This is particularly relevant in chronic disease [80] in which increased density of nerve fibers [81] and enhanced peripheral sensitization play a role and can contribute to a decreased response to antipruritic therapy. Thus, keratinocytes are far from being a physical inert barrier, and they are an integral part of a cross talk with the nervous system and the immune cells.
8. Bacteria and Atopic Dermatitis
Much progress has been made in our understanding of the microbiome in atopic dogs and the importance of restoring biodiversity. We appreciate the role of the microbiome in modulating immunologic responses in dogs and how a dysbiosis can contribute to the development of allergic and inflammatory diseases [74][75]. Decreased cutaneous biodiversity and predominance of
Much progress has been made in our understanding of the microbiome in atopic dogs and the importance of restoring biodiversity. We appreciate the role of the microbiome in modulating immunologic responses in dogs and how a dysbiosis can contribute to the development of allergic and inflammatory diseases [82,83]. Decreased cutaneous biodiversity and predominance of Staphylococcus is a feature of atopic flares [76][77]. As the antibiotic resistance of
is a feature of atopic flares [84,85]. As the antibiotic resistance of Staphylococcus grows and represents a serious challenge for clinicians, we have embraced more topical therapy rather than broad spectrum antibiotics and are acutely aware of how important it is to encourage biodiversity and a healthy sustainable microbiome [78]. Interestingly, in human medicine, topical microbiome transplantation has shown promising results for decreasing the severity of atopic dermatitis and the need for anti-inflammatory therapy [79][80]. In veterinary medicine, several studies have shown a positive effect of probiotic supplementation for modulating immune response in atopic dogs [81] and for decreasing the severity of clinical disease and the need for rescue medications [82].
grows and represents a serious challenge for clinicians, we have embraced more topical therapy rather than broad spectrum antibiotics and are acutely aware of how important it is to encourage biodiversity and a healthy sustainable microbiome [86]. Interestingly, in human medicine, topical microbiome transplantation has shown promising results for decreasing the severity of atopic dermatitis and the need for anti-inflammatory therapy [87,88]. In veterinary medicine, several studies have shown a positive effect of probiotic supplementation for modulating immune response in atopic dogs [89] and for decreasing the severity of clinical disease and the need for rescue medications [90].
9. Take Home Message on Canine Atopic Dermatitis
In summary, it is clear that many different factors play a role in shaping the immune response in canine atopic dermatitis and that keratinocytes and the skin microbiome play a crucial role in modulating the immune response to allergens and modulating inflammation and pruritus. Thus, our management needs to be multimodal with the intent to restore skin barrier and biodiversity while we provide relief from the itch and work toward the long-term re-education of the immune system by using allergen-specific immunotherapy. Each patient will have different thresholds and needs for treatment which may change over the life time of the patient and throughout the course of the year, as flare factors lower the threshold of diseases. Thus, the management of these patients becomes an art of applying our current evidence-based information and tailoring it to the individual needs of our patients. Importantly, we should strive to implement a proactive approach that is suitable for the specific case to minimize the need for rescue medications and the development of secondary infections as part of our efforts to minimize the need for systemic antibiotics.